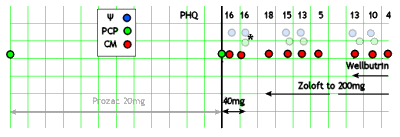
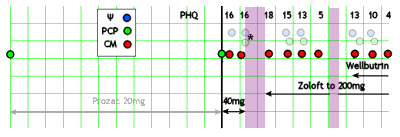
In bygone days, as a resident and as a director of a busy city/county hospital psychiatric emergency room, I had years of working in collaboration with staff and trainees who were the primary contact person. With many cases, I saw the patient in person only briefly. And as a supervisor, I met with residents who brought the cases they needed help with/ Even now, I wish I had support staff in the clinic where I work but alas, it’s just me. Even with all of that experience "collaborating," I have a visceral negative reaction to what I read about the modern version of collaborative care. So when I ran across this recent APA Report on the model, I decided to spend some time looking it over to see if my reflex reaction could be softened up a bit. It’s 85 pages and is written in a salesmanship style with lots of jargon and slogans. I’ve included the intro below. There’s a case reported scatted throughout the narrative, and I’ve extracted much of it at the end, along with a rough diagram [as accurate as the narrative would allow]:
THE COLLABORATIVE CARE MODEL
AMERICAN PSYCHIATRIC ASSOCIATION
ACADEMY OF PSYCHOSOMATIC MEDICINE
Spring 2016
There is expert consensus that all effective Collaborative Care Models share four core elements: [1] team-driven, [2] population-focused, [3] measurement-guided, and [4] evidence-based. These four elements, when combined, can allow for a fifth guiding principal to emerge; accountability and quality improvement. Table 1 reviews the core elements of Collaborative Care implementation. Collaborative Care is team-driven, led by a PCP with support from a "care manager" [CM] and consultation from a psychiatrist who provides treatment recommendations for patients who are not achieving clinical goals. Other mental health professionals can contribute well to the Collaborative Care Model. Collaborative Care is population-focused, using a registry to monitor treatment engagement and response to care. Collaborative Care is measurement-guided with a consistent dedication to patient-reported outcomes and utilizes evidence-based approaches to achieve those outcomes. Additionally, Collaborative Care is patient-centered with proactive outreach to engage, activate, promote self-management and treatment adherence, and coordinate services.
| Table 1: Essential Elements of Collaborative Care |
| Team-Driven: A multidisciplinary group of healthcare delivery professionals providing care in a coordinated fashion and empowered to work at the top of their professional training. |
| Population-Focused: The Collaborative Care team is responsible for the provision of care and health outcomes of a defined population of patients |
| Measurement-Guided: The team uses systematic, disease-specific, patient-reported outcome measures (e.g., symptom rating scales) to drive dinical decision-making. |
| Evidence-Based: The team adapts scientifically proven treatments within an individual clinical context to achieve improved health outcomes. |
The patient had been seen a year earlier by his PCP [Primary Care Physician] and given Prozac for depression with a "fair" response. This time, he was identified on a medical visit because of his score on a waiting-room PHQ-9. The PCP doubled his Prozac to 40 mg/daily and introduced him to the CM [Care Manager]. She obtained a history of his depressive symptoms and something else:
John has recently moved out of his house, and he and his wife are separating. He is staying with a friend in town…
After a month on the higher dose of Prozac, the patient stops it because of jitters. They’re following him with the PHQ-9 and it goes up. The Psychiatrist suggests changing to Zoloft and has a phone conversation with the PCP about titration [the only direct contact between the Psychiatrist and the PCP recorded]. The patient doesn’t fill the Rx, and a month later, he’s worse. Contacted by the CM, he finally starts the Zoloft and the dose is gradually increased. After several months, the patient is feeling better and he and his wife are "fighting less." 5½ months after the initial contact, he’s back with his wife and feels fine [PHQ-9 is 5]. But two weeks later, he stops the Zoloft, and some of symptoms return and persist, in spite of restarting the Zoloft at a maximum dose. The psychiatrist suggests adding Wellbutrin and later increases the dose. At 9½ months from first contact, he is better and his PHQ is 4.
I think reading it at least helped me clarify my negative reactions:
-
The psychiatrist never saw the patient, and as best I could tell only had one direct contact with the PCP. If the PCP had contact along the way when changing the medications around, it wasn’t apparent. It appears that contact was through the CM [though surely that’s not right with changing drugs and titrating doses?].
-
A PHQ-9 and some comorbidity screening don’t a diagnosis make [did they actually make a diagnosis?].
-
A PHQ-9 is hardly a precise clinimetric. I’d prefer asking, and I’m pretty sure patients prefer being asked.
-
There are two instances where a high dose SSRI is stopped with no taper. Both suspect for withdrawal [particularly the first] but were interpreted as worsening!
-
What about "John has recently moved out of his house, and he and his wife are separating. He is staying with a friend in town..." Have we just forgotten that a loss like that can cause all of these symptoms? What were the details? Who left who? Is there a clinician in the house?!
-
Given that he got ill when they separated and got better when he moved back in, so I doubt that the Zoloft had much to do with anything [except maybe withdrawal]. It could he that he was just plenty glad to be home…
I realize they can’t put everything in a summary, and I’m Monday Morning Quarterbacking here, but the things I’ve listed are hardly subtle points.
Robert Whitaker, one of psychiatry’s major critics had this to say about where he thought psychiatry might head in the future [see still around…]:
… So I don’t believe it will be possible for psychiatry to change unless it identifies a new function that would be marketable, so to speak. Psychiatry needs to identify a change that would be consistent with its interests as a guild. The one faint possibility I see – and this may seem counterintuitive – is for psychiatry to become the profession that provides a critical view of psychiatric drugs. Family doctors do most of the prescribing of psychiatric drugs today, without any real sense of their risks and benefits, and so psychiatrists could stake out a role as being the experts who know how to use the drugs in a very selective, cautious manner, and the experts who know how to incorporate such drug treatment into a holistic, integrated form of care. If the public sees the drugs as quite problematic, as medications that can serve a purpose – but only if prescribed in a very nuanced way – then it will want to turn to physicians who understand well the problems with the drugs and their limitations. That is what I think must happen for psychiatry to change. Psychiatry must see a financial benefit from a proposed change, one consistent with guild interests.
So even Robert Whitaker sets a higher mark than this APA Collaborative Care piece. I recognize that they’re trying to streamline things, cut costs, etc. But how can one demonstrate any expertise without ever talking to the patient? Who’s going to look for withdrawal, akathisia? Who’s going to say "What’s going with you and your wife?" Who is going to take the history in this system? Make a diagnosis? What is the place of "psycho-social" in any of this? Did we do John J. any favors with our waiting room screening? Well, here’s the essence of the case narrative below. I’d recommend reading this jargon-filled report before signing any contracts [and be sure to renew your malpractice insurance]…
John J.
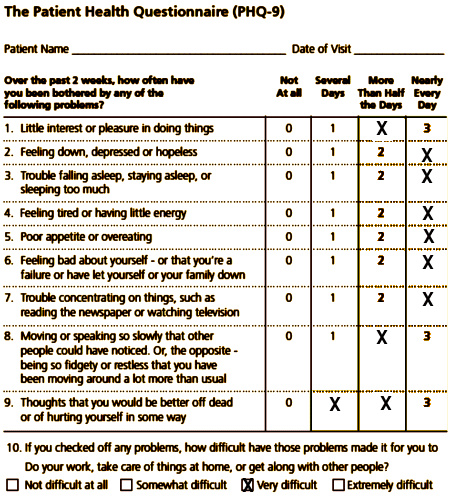
John J. is a 48-year-old white male visiting his PCP, Dr. Stevens, for a follow-up visit for managing hypertension. During the visit, John’s PHQ-9 score is taken and found to be 16, in the moderate range for major depression. John was treated by Dr. Stevens 12 months ago for depression and remains on fluoxetine 20 mg daily, to which he had a fair initial response. This is John’s first PHQ-9, part of the new Collaborative Care protocol instituted by Dr. Stevens’s clinic.
Dr. Stevens discusses the test results briefly with John during their clinic appointment and introduces him to Ms. Cook, a CM/behavioral health specialist with the clinic’s Collaborative Care team. Ms. Cook is immediately available in the clinic to meet patients coming and going from appointments at the request of the PCP or other clinic staff. John agrees to speak with Ms. Cook after the appointment, and Ms. Cook runs through a few patient screens for behavioral health and substance use conditions that are often comorbid with major depressive disorder. John screens negatively for alcohol use or a history of mania. Ms. Cook discovers that John has recently moved out of his house, and he and his wife are separating. He is staying with a friend in town, and it has been hard for him to make it to work consistently. He often goes to bed late and sleeps in, missing his alarm in the morning, and eventually calls in sick. Ms. Cook shares some of this initial information with Dr. Stevens after their appointment, and Dr. Stevens increases John’s fluoxetine to 40 mg daily. She also engages him in a behavioral activation strategy to improve his mood that includes getting together with his friend Joe over the weekend.
Three days later, Ms. Cook has her weekly meeting with Dr. Brown, the consulting psychiatrist. They discuss John, the new addition to Ms. Cook’s caseload. Dr. Brown acknowledges the PHQ-9 score and the fluoxetine increase and reminds Ms. Cook of additional brief intervention techniques she has reviewed in the past with other patients. Five weeks later, during their caseload review, Dr. Brown notices John’s PHQ-9 score is unchanged. Ms. Cook notes that he stopped taking the fluoxetine the week before because of some ongoing jitteriness. Dr. Brown recommends switching to sertraline instead, and Ms. Cook conveys the recommendation to Dr. Stevens by flagging him in the electronic health record. Dr. Stevens reviews John’s other medications the following day and writes a prescription for sertraline after Ms. Cook has called John to discuss the recommendations of the consulting psychiatrist. John agrees to try the sertraline. Ms. Cook reviews the side effects with John and offers her contact information in addition to Dr. Stevens’s office if he has any problems with the medication. Dr. Stevens phones Dr. Brown and asks about the titration schedule of sertraline and starting dosage to confirm his management is appropriate. They agree to continue with increases in this medication with a target PHQ-9 of less than 5 if possible.
Five weeks after his last appointment, John remains depressed. He did not return Dr. Stevens’s last call regarding some recent lab results, and he no-showed one appointment. During their weekly caseload review, John is eighth on Ms. Cook’s list of 58 patients when sorted by PHQ-9 score severity which leads to a case review. Their registry of patients also has flagged John’s PHQ-9 as overdue and above their target. As she and Dr. Brown are reviewing all the patients, they review John’s score and with the information in the registry are able to quickly recall his latest treatment plan, including the sertraline recommendations. Dr. Stevens did write the prescription, but Ms. Cook is unsure what happened after that. She attempted to call John about 1 week after the sertraline was prescribed and left him a message that wasn’t returned. Ms. Cook and Dr. Brown agree that John needs increased outreach given his recent depression and lack of engagement, and Ms. Cook takes on this task over the next week. They then move on to Sue after spending about 5 minutes discussing John…
The following day, Ms. Cook writes a letter from the clinic to John offering assistance and begins to call more frequently. Three days later, John calls back, and he discloses that he never picked up the sertraline and was not sure he was worth the attention of the team. He reports that he didn’t want to feel like a failure again or let anyone down. John’s PHQ-9 score over the phone is 18, and Ms. Cook screens John for suicidal ideation, which is negative. She provides some education around depressive symptoms, the role of the team, and their desire to help him feel better. John agrees to pick up the sertraline from the pharmacy and check-in with Ms. Cook before the weekend to report on how he’s tolerating it.
John, the patient, calls Ms. Cook, the CM, on Friday and reports that he picked up the sertraline and is taking it without side effects but doesn’t feel much different after 2 days. Ms. Cook reassures John that this is not unusual, and that he needs to stick with the medication for 4-6 weeks at the right dose sometimes before his mood may change. They make a plan to check in once a week. In 4 weeks, John’s PHQ-9 score has gone from an 18 to a 15, and he is tolerating the sertraline without any problems. Dr. Brown, the consulting psychiatrist, recommends they titrate the dose to a higher level and continue to monitor John’s response. Dr. Stevens, the PCP, writes a new prescription for John; Ms. Cook confirms that he picks it up at the pharmacy and takes it; and after another 4 weeks, his PHQ-9 is 13. John reports that he is feeling better and has applied for a new job. He and his wife are fighting less, and they are talking about having him move back in. In spite of these gains, however, Ms. Cook discusses John’s remaining symptoms of prominent guilt and negative self-worth and poor quality sleep, energy, and concentration coupled to overeating—all of which contribute to his current score. They formulate a plan to begin more regular exercise. Because his PHQ-9 is still above 5, Dr. Brown’s advice is to continue to titrate the sertraline to the maximum daily dosage, noting his steady improvements.
Four weeks later, John’s PHQ-9 score is 5. He reports that he feels like his old self again, has moved back in with his wife, is exercising more regularly now, and starting to lose some excess weight. Two months after John achieved early remission from his depression, Ms. Cook calls him for a routine check-in. He notes that he stopped taking the sertraline for a couple of weeks right after their last conversation and had a relapse of some of his symptoms. His PHQ-9 score has jumped from 5 to 13, and John is feeling embarrassed and shameful.
He resumed his sertraline at 200 mg about a month ago but still struggles with energy and has stopped his workout routine. Dr. Brown suggests that they augment the sertraline with bupropion, and Dr. Stevens writes the prescription for John. One month later, John’s PHQ-9 score is 10, and Ms. Cook engages him with Behavioral Activation focused on his exercise regimen again. They discuss the cycle of inaction, guilt, and depression, and John agrees to experiment with a different workout regimen and assess his mood. Dr. Stevens automatically adjusts his bupropion to a higher level since he is tolerating it well, and 1 month later John’s PHQ-9 score is 4.


 algorithm for just that problem [see the
algorithm for just that problem [see the 
 I hope I can find a way to stay on for a while because I’ve really enjoyed my time there. It has been a good end-of-career experience. It’s an antidote to some of the disillusioning things I encounter in writing this blog. It’s a mixture of all the different ways of approaching problems help-seeking patients show up with – doing what can be done in the time alloted. I often think of the title of Adolf Meyers’ collected works "Common Sense Psychiatry" – because that’s what it comes down to. Many of the patients I see have outrageous biographies, yet have put together meaningful lives against the odds. And even with the brief and infrequent visits to the clinic, I’m often awed with the work that can be accomplished. And it’s good for a psychoanalytically oriented psychotherapist to work in a situation where the very medications I talk about here as overused can be so helpful in treatment if you’re careful.
I hope I can find a way to stay on for a while because I’ve really enjoyed my time there. It has been a good end-of-career experience. It’s an antidote to some of the disillusioning things I encounter in writing this blog. It’s a mixture of all the different ways of approaching problems help-seeking patients show up with – doing what can be done in the time alloted. I often think of the title of Adolf Meyers’ collected works "Common Sense Psychiatry" – because that’s what it comes down to. Many of the patients I see have outrageous biographies, yet have put together meaningful lives against the odds. And even with the brief and infrequent visits to the clinic, I’m often awed with the work that can be accomplished. And it’s good for a psychoanalytically oriented psychotherapist to work in a situation where the very medications I talk about here as overused can be so helpful in treatment if you’re careful.