Posted on Tuesday 17 June 2014
Huffington PostBy The Canadian Press12/06/2013The Conservative government has introduced new legislation aimed at protecting consumers from unsafe medications and reducing adverse drug reactions. The Protecting Canadians from Unsafe Drugs Act is known as Vanessa’s Law in honour of the late daughter of Conservative MP Terence Young. The 15-year-old died of a heart attack 13 years ago while on a prescription drug for a stomach ailment. The medication was later deemed unsafe and pulled from the market.
Young, MP for Oakville, has been fighting ever since for a more stringent Canadian drug-monitoring system. Under the new legislation, the government now has the power to initiate mandatory recalls for unsafe drugs and to demand reports from health-care institutions on adverse drug reactions. The bill also allows the government to impose tough new penalties for unsafe products, including jail time and new fines of up to $5 million a day instead of the current $5,000.
Drug companies must also revise labelling to provide details on health risks, and to do further testing on medications when they are shown to pose dangers to some consumers, especially children.
Health Canada posts Diane-35 Safety Review Summary and works with partners on a prescriber checklist as first stepCNWfrom Health Canada04/08/2014The Honourable Rona Ambrose, Minister of Health, today delivered on a commitment to begin transparently publishing drug safety reviews. The Minister committed to providing Canadians with access to Health Canada drug safety reviews with the posting of a summary of the safety review of the acne treatment Diane-35. This is the first summary safety review to be made public.
Once a drug is on the Canadian market, Health Canada continues to monitor the safety of health products to identify and assess potential harms. As a result, departmental safety reviews are conducted when a safety issue is identified for a product on the Canadian market. The posting of summary safety reviews will provide Canadians with plain-language descriptions of Health Canada’s findings and decisions, so patients can make informed decisions and continue to have confidence in the health products they use. The full reports will be made available through a link on Health Canada’s website; however they are subject to redactions of personal and confidential information.
The posting of summary safety reviews is the first in a series of measures that Health Canada is taking to be more transparent and open with Canadians about regulatory decisions. As part of Health Canada’s Regulatory Transparency and Openness Framework, the department has committed to providing Canadians with credible and timely information. This easy to understand information will allow them to make well-informed decisions concerning their health and that of their families. Canadians will also have the chance to provide Health Canada with feedback on the Framework.
Health Canada has also made available today a practical new tool to assist in the safe prescribing of Diane-35 and to better inform healthcare professionals and patients of its risks. In consultation with the Canadian Pharmacists Association, the Canadian Dermatology Association, and the Institute for Safe Medication Practices, Health Canada has developed a checklist to guide healthcare professionals through the decision to prescribe Diane-35. The checklist will reinforce existing warnings and precautions for this drug.
Today’s announcements build on the Government’s Patient Safety agenda, including Legislation that was introduced in December 2013, known as the Protecting Canadians from Unsafe Drugs Act (Vanessa’s Law).
Amended Vanessa’s Law advances transparency commitmentsDigital Journalby Canada NewsWireJune 16, 2014The Government of Canada welcomes amendments that would strengthen transparency in the proposed patient safety legislation Vanessa’s Law [Bill C-17]. Since introduction in December 2013, Vanessa’s Law has received broad support from members of Parliament, stakeholders and healthcare groups in recognition of the important drug safety improvements that it would deliver, should it become law.
The amendments to Bill C-17 were introduced by Member of Parliament, Terrence Young and adopted by the House of Commons Standing Committee on Health on June 12. The Bill has now passed Third Reading in the House of Commons and moves to the Senate for consideration. The amendments include the requirement that both positive and negative decisions about drug authorizations be disclosed on a public website; and that clinical trial information be disclosed on a public registry. The amendments also better define the scope of confidential business information [CBI] and allow the Minister to disclose CBI about a product if the Minister believes the product may pose a serious risk to Canadians.
The amendments to Bill C-17 would enable Health Canada to continue strengthening its Regulatory Transparency and Openness Framework that was announced in April of this year by the Minister. The Framework commits Health Canada to a set of concrete initiatives that would make easy to understand regulatory health and safety information more available to Canadians. With this information, Canadians can make well-informed decisions concerning their health and that of their families.
Quick Facts
 Unless I’ve misread this story, it’s aiming towards being a model for us all. The details of the Data Transparency scheme aren’t completely apparent, but the progression of their march is encouraging and with a track record like this, one would predict that even if they falter on the details, they’ll get things right sooner or later [if I’m wrong about that, I hope some of our neighbors in Canada will let me know]. I particularly like the idea of posting the public summaries. I hope the specifics live up to my optimism…
Unless I’ve misread this story, it’s aiming towards being a model for us all. The details of the Data Transparency scheme aren’t completely apparent, but the progression of their march is encouraging and with a track record like this, one would predict that even if they falter on the details, they’ll get things right sooner or later [if I’m wrong about that, I hope some of our neighbors in Canada will let me know]. I particularly like the idea of posting the public summaries. I hope the specifics live up to my optimism…


 worry [see
worry [see 
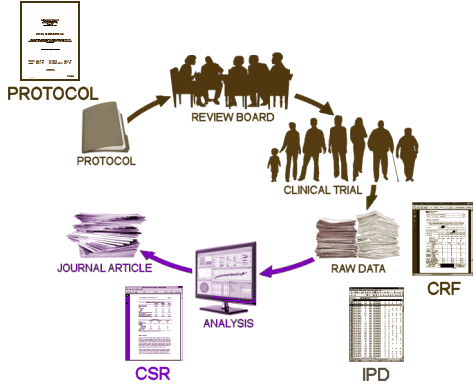
 Notice that they mention "… a series of meetings in Brussels, organised jointly by PhRMA and EFPIA, in the week of August 26 to advance these strands." That was that incendiary presentation by AbbVie’s lawyer, Neal Parker [see
Notice that they mention "… a series of meetings in Brussels, organised jointly by PhRMA and EFPIA, in the week of August 26 to advance these strands." That was that incendiary presentation by AbbVie’s lawyer, Neal Parker [see