I really have a distaste for grant applications. I did some when I was an NIH Fellow in the bronze age and much later wrote periodic training grants. I get it why one has to fill out all that stuff and look endlessly at the endless guidelines, but getting it doesn’t erase the tedium of doing it. I am discovering that reading such things is an equally odious task, having tried yesterday to make some sense out of the NIMH RAISE Studies I mentioned earlier [a fabrication?…]. While I’m at it, I apologize for the disjointedness of that last post. I started out with something in mind, but every time I looked something up, some new confusion reared its head. So the post wandered as I wandered from place to place trying to figure things out. This post is a second look at the material from that post.
First off, I declare that I’m a supporter of both the efforts to identify people who are going to have psychosis in advance, though I question whether the existing methods being proposed are on the right track. And I also believe that early intense intervention in FSE [First Schizophrenic Episode] is an imperative. So my interest in the RAISE Programs isn’t antagonistic, I just want to be sure that what’s being proposed is rational and likely to get us somewhere useful. So first, here is what I could find out about the grants:
NIMH Director’s blog
By Thomas Insel
December 29, 2009
From the extraordinary funding opportunities presented by the passage of the American Recovery and Reinvestment Act of 2009 [Recovery Act] to significant new investments in research and resource infrastructure — this has been a remarkable year for our Institute. I would like to reflect with you on how the work of 2009 has prepared us for the year ahead…
Finally, I would like to highlight a research project launched this year that seeks to fundamentally change the way schizophrenia is treated: the Recovery After an Initial Schizophrenia Episode [RAISE] project. The RAISE project, which is also supported through Recovery Act funds, will develop and test innovative and coordinated intervention approaches in the early stages of the illness when symptoms may be most responsive to treatment. The study is designed to alter the long-term disability that can result from schizophrenia and help ensure that people with the disease can lead productive, independent lives. Importantly, the interventions being tested will be designed from the outset to be readily adopted in real-world health care settings and quickly put into practice.
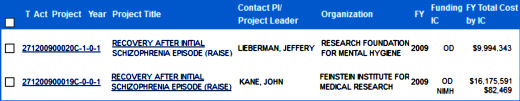
and here are the grants:
[reformatted to fit]
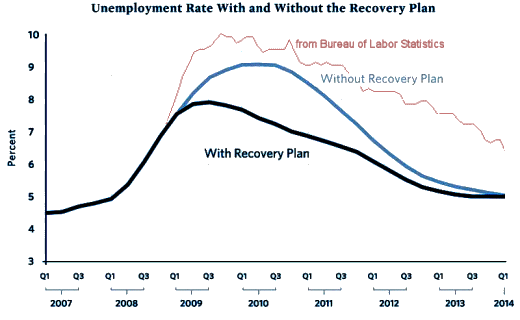
OD stands for Office of the Director, in this case I presume that the Director is allocating the funds from the American Recovery and Reinvestment Act [ARRA]. An Aside: This was the stimulus money after the Great Recession of 2008, I can’t resist showing their prediction graph from that time with what actually happened added [in red].
So with the ARRA windfall, Dr. Insel funded two RAISE [Recovery After an Initial Schizophrenia Episode] grants: RAISE Connection [271200900020C-1-0-1] and RAISE ETP [271200900019C-0-0-1]. Dr. Jeffrey Lieberman was Principle Investigator on RAISE Connection – a two site effort looking to recruit >370 subjects. It looks as if they planned to have two treatment arms assigned randomly at both sites. Dr. John Kane [1][2] was the Principle Investigator on RAISE ETP with 33 sites with a projected enrollment of >400 also with two arms, but each site was assigned to a specific arm. While the NIMH grant description for both was the same, the clinicaltrials.gov descriptions differed and they followed a remarkably different revision history.
And these are the two studies that were implemented from these grants:
-
Behavioral: Multi-element, team-oriented treatment
Arm Label: Team-based treatment:
Participants will receive treatment and services based on their needs coordinated by a small team that is led by a clinical coordinator. Services that are available to the participants include social skills training, medication treatment to address symptoms, education and employment advising, and substance use treatment.
-
Behavioral: Connections to community-based treatment and services
Arm Label: Facilitated community-based treatment:
A care specialist will work with participants to identify and link to treatment and service options that are available in the community. These options can include finding a therapist, obtaining ongoing medication treatment to address symptoms, joining a support group, and getting a job coach.
-
Dr. Lieberman was replaced as the responsible party by Susan Ellis PhD.
-
The target enrollment was dropped from 370 to 80, and shortened.
-
The active comparator was dropped [now only one arm]:
"The Connection Program aims to to assess the effectiveness of a Team-based intervention for individuals with a first psychotic episode, observing outcomes over time for our study participants. When tracking outcomes, the Connection Program will make comparisons with what is known about the natural history of untreated first episode of psychosis as well as usual care outcomes from other experimental studies…"
-
The study was logged in as recruiting again.
-
Obviously, it was no longer randomized or blinded as there was only one group.
Thereafter, Lisa Dixon MD replaced Susan Essock PhD, and on
04/16/2013, the enrollment was dropped to 65. The study was completed on 12/23/2013.
RAISE ETP [Extended Treatment Program]: Clinical Trial NCT01321177 was registered on January 27, 2011 with two arms:
-
Behavioral: Integrated Treatment
Arm Label: Integrated Treatment:
Integrated program of treatments and services delivered by a coordinated team of providers that includes:
-
education about schizophrenia and its treatment for the participants and their family members
-
medication for symptoms and preventing relapse that uses a computerized decision support system
-
strategies for managing the illness and building personal resilience
-
help getting back to school or work using a supported employment/education model
-
Behavioral: Connections to community-based treatment and services
Arm Label: Facilitated community-based treatment:
A care specialist will work with participants to identify and link to treatment and service options that are available in the community. These options can include finding a therapist, obtaining ongoing medication treatment to address symptoms, joining a support group, and getting a job coach.
Standard mental health treatments and services offered at the local agency that may include:
It was logged as recruiting on 03/22/2011 and as Active, not recruiting on 12/27/2012.
If you’ve made it through this tediousness, you can see that something fairly dramatic happened along the way. RAISE Connection got off to the first start but shortly after they started recruiting, they stopped. Then a few months later, it was as if it just shut down. There was a new P.I.; they dropped the Active Comparator; they radically downsized the projected enrollment; then sauntered along with changing investigators and sponsors; and they ended up with only 65 subjects. At the same time RAISE Connection went on ice, RAISE ETP got started and barreled ahead full bore. I can locate nothing that tells me what happened, but I think it matters some. RAISE Connection was awarded ~$10 M and basically shut itself down when RAISE ETP got started. So I think it’s legitimate to ask, Where did that $10 M go?
There have been two windfall moments in this story. First, the availability of ARRA funding that got both studies started, granted by the Office of the Director outside the usual NIMH channels. One study,
RAISE Connection, seems to have fizzled [
Where did that $10 M go?]. The other appears to be perking along. The next windfall was the recent availability of
SAMHSA Block Grant opportunities for the States to implement FSE programs. There is a complex and detailed document,
Evidence-Based Treatments for First Episode Psychosis: Components of Coordinated Specialty Care, from both
RAISE studies on the NIMH site that summarizes all the information the NIMH has extracted to pass on to the States applying for these block grants listing all of their various supplementary materials. I’m not up to taking it on just yet, but I did look at the acknowledgements at the end:
Portions of this report have been drawn from the final reports of the RAISE Connection Program [Contract No. HHSN271200900020C] and interim progress reports of the RAISE Early Treatment Program [Contract No. HHSN271200900019C]. We thank the following subject matter experts for their careful review of this document, insightful feedback, and helpful editorial suggestions: Lisa Dixon, Susan Essock, and Howard Goldman [RAISE Connection Program]; John Kane, Nina Schooler, Delbert Robinson, Jean Addington, Mary Brunette, David Penn, and Patricia Marcy [RAISE Early Treatment Program]; Kristin Cadenhead, Barbara Cornblatt, Keith Nuechterlein, Diana Perkins, and Scott Woods. Finally, we thank Patrick McGorry [AO, M.D., Ph.D, FRCP, FRANZCP] for generously sharing resources developed in Australia to support broad implementation of evidence-based treatment approaches for FEP, as well as lessons learned from the national roll out of coordinated specialty care programs in that country.
Dr. Insel’s blog post, Director’s Blog: From Research to Practice, focused of the rapidity of the NIMH translation of the RAISE Connection study to the SAMSA Block Grants for the States to implement FSE/FPE programs. This is a relative huge initiative similar to the EPPIC program in Australia. First off, the RAISE Connection study is absolutely nothing to write home about, and we don’t have access to "the final reports of the RAISE Connection Program … and interim progress reports of the RAISE Early Treatment Program" to allow us to know if the results of these programs justify their being a template for such a major undertaking. It might be the greatest idea in the world or it might be a major dud and a massive waste of resources.
Again, I am on the side of a well funded program for an intense focus on the early days of psychosis including psychosocial interventions. If we’re going to have an impact on the course of this illness, that’s the time in the course of things to get started – and those patients need a lot more that just medications. I don’t question the credentials of either Dr. Lieberman or Dr. Kane to lead that effort. But this is a time for data transparency – and what we have is too little to help us decide for ourselves. Are we moving ahead based on solid evidence, or because the money’s available? I can’t determine the answer to that so far with what we have to look at, and the days for this kind of expensive conclusion without public access to the the data justifying that conclusion should be behind us. I would call on Dr. Insel to make those documents mentioned above available for all of us to see – and while he’s at it, answering the questions about Raise Connection – "Where did that $10 M go?"
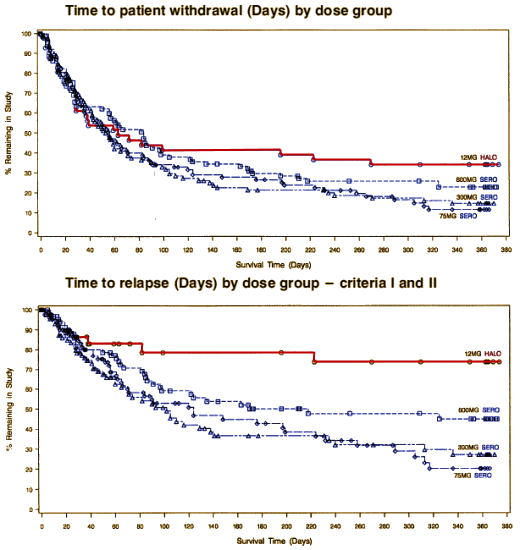
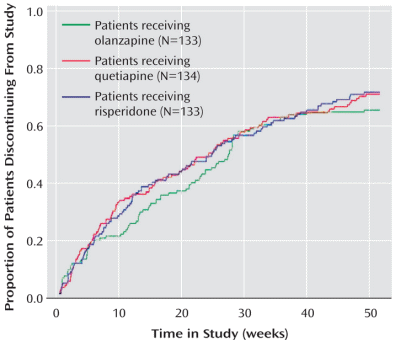 Obtaining that statement and this graph for the AstraZeneca marketing department to counter the other studies showing up Seroquel® [quetiapine] as a weak sister was the goal of the C.A.F.E. study. How can I say that without even being tentative? It’s easy, AstrZeneca said it themselves. Back in 1997, Andrew Goudie, a researcher who had previously worked on Seroquel® wrote Jeffrey Goldstein of the AstraZeneca’s R&D section requesting funding for a further study. In the return email, Goldstein made their policies very clear:
Obtaining that statement and this graph for the AstraZeneca marketing department to counter the other studies showing up Seroquel® [quetiapine] as a weak sister was the goal of the C.A.F.E. study. How can I say that without even being tentative? It’s easy, AstrZeneca said it themselves. Back in 1997, Andrew Goudie, a researcher who had previously worked on Seroquel® wrote Jeffrey Goldstein of the AstraZeneca’s R&D section requesting funding for a further study. In the return email, Goldstein made their policies very clear:



 A few years back, I wrote a post [
A few years back, I wrote a post [ A remote desktop is a single window whose contents can’t be removed or operated on by any other program in your computer. So everything you do has to be contained within that single window, and any operations have to be done by the limited software provided inside that window. It’s a royal pain in the ass to try to work with the thousands of pages in a clinical trial in that environment. As part of a group trying to vet Paxil Study 329 using this tool, I can personally attest to the fact that it’s an obstruction extraordinaire. It’s like going to sea to see the world in a submarine looking through a periscope.
A remote desktop is a single window whose contents can’t be removed or operated on by any other program in your computer. So everything you do has to be contained within that single window, and any operations have to be done by the limited software provided inside that window. It’s a royal pain in the ass to try to work with the thousands of pages in a clinical trial in that environment. As part of a group trying to vet Paxil Study 329 using this tool, I can personally attest to the fact that it’s an obstruction extraordinaire. It’s like going to sea to see the world in a submarine looking through a periscope.  Simply put, this is just yet another trick to maintain ownership of data for no rational reason. The only purpose is to maintain control, and the only reason to do that is to be able to cheat, and cheat they did in spades. Did AbbVie withdraw their suit by extracting this deal from the EMA? My guess would be that the answer is "yes". These people don’t know how to do anything by playing it straight. Fiona Godlee probably said it right [
Simply put, this is just yet another trick to maintain ownership of data for no rational reason. The only purpose is to maintain control, and the only reason to do that is to be able to cheat, and cheat they did in spades. Did AbbVie withdraw their suit by extracting this deal from the EMA? My guess would be that the answer is "yes". These people don’t know how to do anything by playing it straight. Fiona Godlee probably said it right [