by
Anna R. Brandon, Madhukar H. Trivedi, Linda S. Hynan, Paula D. Miltenberger, Dana Broussard Labat, Jamie B. Rifkin, and C. Allen Stringer
Journal of Clinical Psychiatry. 2008 69[4]: 635–643.
Objective: Little is known about depression during pregnancy in women with high maternal or fetal risk, as this population is often excluded from research samples. The aim of this study was to evaluate depressive symptoms and known risk factors for depression in a group of women hospitalized with severe obstetric risk.
Method: In the antenatal unit, 129 inpatients completed the Edinburgh Postnatal Depression Scale [EPDS], the Dyadic Adjustment Scale [DAS], and the Maternal Antenatal Attachment Scale [MAAS] from October 2005 through December 2006. A subset of women were administered the Mood Disorder module of the Structured Clinical Interview for DSM-IV Axis I Disorders [SCID] based upon a score of ≥11 on the EPDS. Obstetric complications were classified according to the Hobel Risk Assessment for Prematurity.
Results: Fifty-seven of the 129 women [44.2%] scored 11 or greater on the EPDS, and at least 25/129 [19%] met the DSM-IV criteria for Major Depressive Disorder [MDD]. Mothers reporting high attachment to the fetus on the MAAS reported lower severity of depressive symptoms [rho=−0.33, p< 0.001]; those reporting interpersonal relationship dissatisfaction on the DAS endorsed higher depressive severity [rho=−0.21, p=0.02]. Severity of obstetric risk was unrelated to depression but, one complication, incompetent cervix, was positively associated with level of depressive symptomatology.
Conclusion: Findings indicate a higher prevalence rate of MDD in women with severe obstetric risk than that reported in low-risk pregnancy samples, suggesting the need for routine depression screening to identify those who need treatment. Fewer depressive symptoms were reported by mothers reporting strong maternal fetal attachment and greater relationship satisfaction.
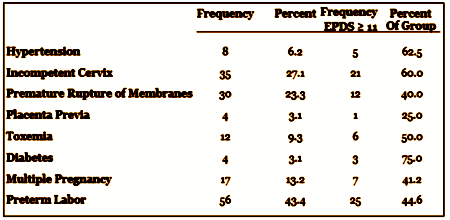
First, the study: Women with high risk pregnancies are usually admitted to the hospital for observation. Here’s a table from the article with the specific risk factors. The study consisted of the subjects completing a group of self report questionnaires. Those with a score of > 11 on the Edinburgh Postnatal Depression Scale [EPDS] had a SCID structured Interview as a marker for the presence of Major Depressive Disorder [MDD]. Here’s the table of reasons for admission from the article:
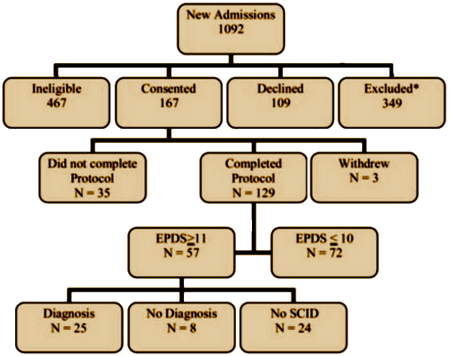
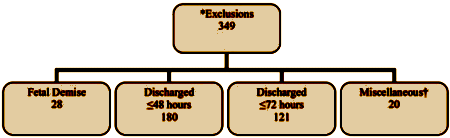
And here’s the consort and flow through the study [modified to fit your screen]:
The findings were:
-
"Fifty-seven of the 129 women [44.2%] scored 11 or greater on the EPDS, and at least 25/129 [19%] met the DSM-IV criteria for Major Depressive Disorder [MDD]"
-
"… the data indicated the proportion of those with MDD was at least 19%, higher than reported in other studies of perinatal women."
In case you haven’t noticed, this is not a candidate for study of the year. They’re using a questionnaire for predicting post-partum depression to screen for pre-natal depression. The fall-off in the flow chart is impressive and with the likelihood of non-random missingness, who knows what the numbers mean. Their control group is other studies – referenced but with findings not shown except in summary:
the best estimate of point prevalence for MDD at any time during pregnancy is 12.7%, with as many as 18.4% reporting major or minor depression.
They seem to know that it’s a shambles, saying:
"Conducting pure research in a hospital unit such as the setting for this study and with a population such as this one is a challenging task. The process of pregnancy and childbirth has always been unpredictable, which is illustrated by the rate of exclusions that occurred because mothers either delivered or were discharged within 72 hours of admission, even when this was not expected."
Fortunately, the fact that it’s so uninterpretable doesn’t interfere with the reason I’m talking about it. It’s not even my more usual reason for looking at such studies: trolling for patients to put on antidepressants. In the conclusion and the implications for further research, they don’t even suggest antidepressants – to their credit. In fact, the first author,
Dr. Anna R. Brandon, has a list of publications including looking into
psychotherapeutic interventions for depressed pregnant patients because they were afraid to take antidepressants. Good for her, and good for them. Nor is this posted here because the results are obvious:
"Corroborating previous work, prenatal depression is significantly associated with psychiatric history, lower maternal fetal attachment, and higher relationship dissatisfaction."
It’s the phrase "met the DSM-IV criteria for Major Depressive Disorder [MDD]" that got it on the record of this blog. These are women towards the end of their pregnancies who found themselves hospitalized for "high risk" at the end of their pregnancy. That means:
"… a pregnancy may be defined “high risk” on the basis of an increased probability of fetal anomaly, compromises to maternal or fetal health, or significant risk for maternal or fetal demise."
In my book, that’s a stress of major proportions. Were I capable of being in that situation, I’m sure I’d be pretty damned unhappy. The authors knew that. In discussing the difficulties of doing their study, they said:
"The protocol required two face-to-face interviews and the completion of a packet of self-report measures, a process that may have seemed overwhelming to patients already overwhelmed with the implications of their obstetric complications and the effect of hospitalization upon their spouses and families."
In fact, in this cohort, there were more cases of women who lost their babies than women where they could document the "MDD" they were looking for with a structured interview:
Call it The Depression of Dire Straits or The Understandable Misery of carrying a Pregnancy that Might Go Very Badly, but not Major Depressive Disorder. We used to call it Reactive Depression. I liked that term because it was the truth. What about patients who really do have a Melancholic Depression either unrelated to the pregnancy or precipitated by the pregnancy? You don’t need a screening instrument for that. The clerk doing the intake can see it with the naked eye. If Major Depressive Disorder is a Disorder, don’t stick women in this situation with that label. All you’ll do is muck up their insurance premiums in perpetuity. But even though this group didn’t go down the antidepressant path, here’s an example of what is happening in the real world:
by Jeffrey R. Lacasse and Joanne Cacciatore
Death Studies doi:10.1080/07481187.2013.820229
To examine psychiatric prescribing in response to perinatal/neonatal death, we analyzed data from a cross-sectional survey of 235 bereaved parents participating in an on-line support community. Of the 88 respondents prescribed medication, antidepressants were most common [n = 70, 79.5%] followed by benzodiazepines/sleep aids [n = 18, 20.5%]. Many prescriptions were written shortly after the death [32.2% within 48 hours, 43.7% within a week, and 74.7% within a month]. Obstetrician/gynecologists wrote most prescriptions given shortly after loss. Most respondents prescribed antidepressants took them long-term. This sample is select, but these data raise disturbing questions about prescribing practices for grieving parents.
The current usage of the diagnosis Major Depressive Disorder [MDD] trivializes human experience by implying that very unhappy or symptomatically depressed is a disease or a disorder. This is a case where Thomas Szasz is totally correct. When the DSM-III conflated the magnitude of the felt symptoms with the presence of psychiatric conditions that are, in fact, clinical entities with unique histories and course, the framers made an error that has had widely felt consequences that we’re all aware of – popularized by some psychiatrists [one of whose names in on this paper – Madhukar Trevidi]. I’ve been convinced by others that this was not Robert Spitzer’s intent, but it doesn’t change today’s problematic reality. Likewise, the widely used term antidepressants for certain of our medications seems benign, but it’s not. In general medicine, that means "give them to unhappy sad depressed people" [we don’t call antihistamines anti-sneezers, or antacids anti-upset-tummiers]. The antidepressants are not effective in helping people with the human responses to adversity, at least not in my hands or in our literature. But they can, at times, add to the burden.
If the intent of the authors of the first paper is to highlight the fact that the threat of fetal loss is a big deal in the life of a mother, even a reluctant mother, more power to them. I’d only suggest they find another term for it. As to the second paper, it highlights the epidemic of inappropriate uses of psychiatric medications everywhere, and the even worse fact that routinely, their use becomes long term. I’ll probably get myself in trouble with the authors saying this, but in acute bereavement, sometimes the most helpful thing in the world is a few good nights of sleep – even that is with the accent on "a few." But a third of them on antidepressants? Absurd.
Fetal or neonatal death, or even its threat, can be a life altering event. What is easily overlooked is that for the mother-to-be, attachment well antedates birth, and it’s an unusual attachment because it’s to what the child will be, what the child will be like in the life of the mother. The well known worried preoccupation of the healthy new mother has been understood as the mother’s adapting to the reality of the child they actually have, transitioning from a relationship with the virtual child. Whatever the meanings, it’s an attachment and a loss like no other, understood best by people who know it from experience, either their own or long exposure to others. And while this is not the format to discuss it, even the threat of a lost pregnancy can have long range consequences for a mother/child pair persisting long after the danger has passed, worthy of exploration beyond symptoms…


 shut down Atlanta Georgia for a week or more. We all bought 4 wheel drive cars and stocked up on supplies after that, but that was a long time ago – so we forgot. We’re sitting in our houses watching television [which plays non-stop monotonous weather stories], burning huge fires in our fireplaces, piddling on the Internet, and eating from those cans in the back of the cupboard that stay there for years, forgotten – things like beets [Yuk]. At least there’s NetFlix…
shut down Atlanta Georgia for a week or more. We all bought 4 wheel drive cars and stocked up on supplies after that, but that was a long time ago – so we forgot. We’re sitting in our houses watching television [which plays non-stop monotonous weather stories], burning huge fires in our fireplaces, piddling on the Internet, and eating from those cans in the back of the cupboard that stay there for years, forgotten – things like beets [Yuk]. At least there’s NetFlix… Increasingly lonely, Wellcome spent his last years preparing for his life’s work to be carried on after his death, for the benefit of mankind. Wellcome grew increasingly lonely in the 1910s and 1920s. He had separated from his wife Syrie in 1910 and one by one his close friends – never many in number – grew old and died. In his early 60s he had no one with whom he could share his deepest interests. Even so, his passion for history and collecting remained undimmed. At a time of life when many would slow down, he orchestrated an archaeological dig between 1911 and 1914 in the Sudan, continued rearranging and collecting for his Historical Medical Museum, and opened a museum on the history of medicine. Meanwhile, he remained very much in control of his company, suggesting avenues of research for his scientific laboratories and adapting his enterprises to the strains of World War I. Such was his industry that a member of staff later commented that “one of the things about Sir Henry was that he never thought he would die.”
Increasingly lonely, Wellcome spent his last years preparing for his life’s work to be carried on after his death, for the benefit of mankind. Wellcome grew increasingly lonely in the 1910s and 1920s. He had separated from his wife Syrie in 1910 and one by one his close friends – never many in number – grew old and died. In his early 60s he had no one with whom he could share his deepest interests. Even so, his passion for history and collecting remained undimmed. At a time of life when many would slow down, he orchestrated an archaeological dig between 1911 and 1914 in the Sudan, continued rearranging and collecting for his Historical Medical Museum, and opened a museum on the history of medicine. Meanwhile, he remained very much in control of his company, suggesting avenues of research for his scientific laboratories and adapting his enterprises to the strains of World War I. Such was his industry that a member of staff later commented that “one of the things about Sir Henry was that he never thought he would die.”
