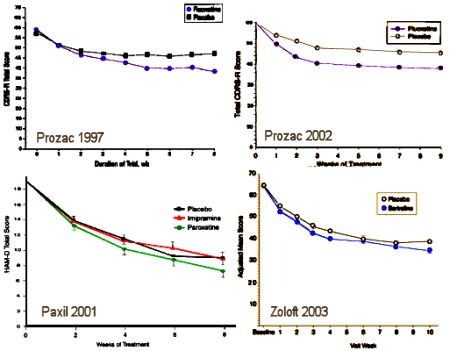
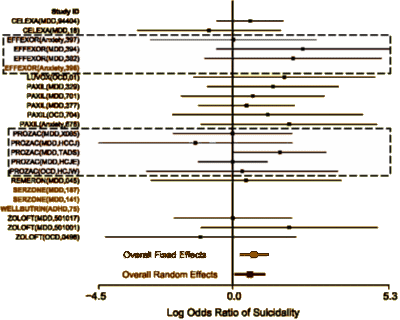
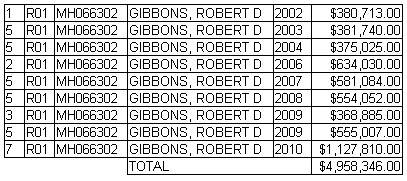
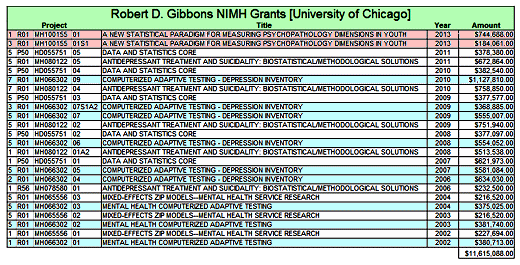
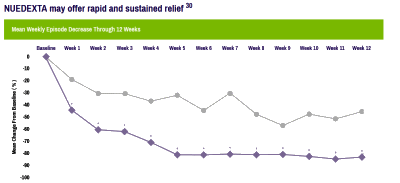
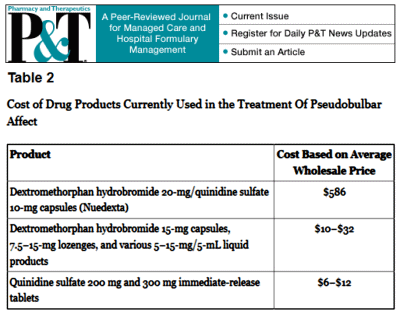
The trajectory of the DSM-5 revision was set in the book, A Research Agenda for the DSM-5, published in 2002 – a time of high hopes for a biomedical basis for psychiatric disorders. The plan was to include biomarkers in the manual and to add dimensional parameters that cut across disorders, getting around the imprecision of the categorical diagnostic entities. While the DSM-III and earlier revisions were widely used by clinicians, researchers were frustrated that neither the research tools nor the psychopharmacologic agents mapped to the defined criteria. In short, they were looking not to just revise the existing manual. They wanted to change it. The book was followed by an elaborate series of conferences, meetings, and presentations to shape the DSM-5 to fit that vision:
By the time They got underway and began the revision process, they were in a different place from where they started. The world had changed. There was an increasing awareness of the extent to which the pharmaceutical industry had invaded psychiatry and that edifice was crumbling. The fabled "pipeline" was headed for extinction. The dreams of a psychopharmacologic and neuroscientific future were met with the reality of scandal, adverse effects, limited efficacy, disappointing discovery, and a pharma bail-out.
The criticism seemed to come from an unusual direction, but in retrospect, it makes perfect sense that the people who had invested themselves in the DSMs and biological research were the ones who were looking. The watchdogs like Drs. Bernard Carroll and Bob Rubin began to look at some of the misbehavior in the publications filling our journals. Whistle-blowers like Allen Jones with TMAP and prosecuters like Elliot Spitzer in New York began to look at false claims and corruption. Civil suits for damages against the pharmaceutical industry became common. And then in 2007, Robert Spitzer who had shepherded the DSM-III and -IIIR focused attention on the DSM-5 process and its secrecy [confidentiality agreements]:
h-madness
by H.S. Decker
04/27/2010
The scenario started simply and privately in April 2007, five years before the revised manual was scheduled for publication. Robert Spitzer, the psychiatrist who had headed the APA’s Task Force that revised the association’s third diagnostic manual in 1980 [ DSM-III], dropped a two-line request to a colleague, Darrel Regier, Vice Chair of the Task Force that is currently updating the Association’s fifth manual [DSM-5]. Would it be possible for Regier to forward to him a copy of the minutes of the Task Force’s first two meetings? Regier answered Spitzer quickly, saying summary minutes would be available to individuals like him for private use, but asking him to wait until the APA Board of Trustees formally approved the membership of the Task Force. After an interval, having received no minutes, Spitzer renewed his request. But nine months passed before Regier gave Spitzer a definitive answer in February 2008: Due to “unprecedented” circumstances, including “confidentiality in the development process,” David Kupfer, Chair of the Task Force, and Regier had decided the minutes would be available only to the Board of Trustees and the Task Force itself…
In a letter to the editor, June 11, 2008, Spitzer began: “The June 6th issue of Psychiatric News brought the good news that the DSM-V process will be ‘complex but open.’” And, he added, just a few weeks before, the outgoing president of the APA had stated that in the development of DSM-V the APA is committed to “transparency.” Then Spitzer expostulated: “I found out how transparent and open the DSM-V process was when [Regier] informed me that he would not send me a copy of the minutes of DSM-V task force meetings … because the Board of Trustees believed it was important to ‘maintain DSM-V confidentially.’” Spitzer then made available in his letter a paragraph from the Acceptance Form that all Task Force and Work Group members had signed stating that during their term of appointment and after, they would not “make accessible to anyone or use in any way any Confidential Information”…
Spitzer continued: “I didn’t know whether to laugh or cry. Laugh – because there is no way Task Force and Work Group members can be made to refrain from discussing the developing DSM-V with their colleagues. Cry – because” to revise a diagnostic manual in secrecy destroys the scientific process, “the very exchange of information that is prohibited by the confidentiality agreement”… Once galvanized into action, he began an unrelenting campaign against the “secrecy” of the fifth DSM revision process and urging “transparency,” soon forcing the APA to defend itself…
The APA did defend itself – but that was all it did. The Task Force remained a closed shop. Dr. Allen Frances, Chair of the DSM-IV Revision, had declined to join Spitzer’s earlier challenge. But in 2009, he too complained about the DSM-5 [
A Warning Sign on the Road to DSM-V: Beware of Its Unintended Consequences]. This time the APA came out swinging with a condescending response that ended with an accusation that Frances was motivated by a COI [
Setting the Record Straight: A Response to Frances Commentary on DSM-V]. And so it went for several years as others joined Dr. Frances challenges to both the process and content of the revision. The responses from the APA and the Task Force were often silence, defensive denial, or attacks. They hired a PR person who called Spitzer and Frances
dangerous men. They set up a website
DSMFacts that published regular refutations to criticisms or negative press. They were more polite to critics from outside psychiatry, but the shop doors remained closed, and they didn’t ever really engage their critics, just reiterated their given position.
Throughout the remaining revision process, the APA and DSM-5 Task Force maintained a "circled wagon" posture and continued along the path set out in 2002 – by this time an anachronism perhaps embraced inside their compound, but few other places. There were crises – having to abandon the planned biomedical additions, a remarkably bad showing in the field trials, widely publicized revolts by the other mental health professions. One such problem was the increasing focus on Conflicts of Interest in psychiatry – particularly at the upper levels. Senator Grassley’s investigations in 2008 brought Conflicts of Interest into more focus, and several chairmen of psychiatry were unseated. The president of the American Psychiatric Association at the time was on that list. What was more disturbing was that Grassley’s charges were of personal entrepreneurialism, failing to report personal outside income to their universities.
In assembling the DSM-5’s Task Force and Work Groups, the APA’s Board of Trustees developed an extensive process of written disclosure of potential conflicts of interest. These disclosures are required of all professionals who participate in the development of DSM-5. An independent APA committee reviews these disclosure documents, which are updated annually or whenever a member’s financial interests change. Individuals are only permitted to serve on a work group or the Task Force if they are judged to have no significant financial interests.
Now the DSM-5 is in print, and we learn that Dr. Kupfer and other colleagues on the DSM-5 Task Force had an entrepreneurial Conflict of Interest of major import that was unreported throughout the period when they kept saying
not a problem – admitted only after being exposed. So far, the APA has responded only with a
memo from the Assembly Speaker that minimizes the impact and doesn’t address the real meaning of this revelation. As I said, it "
reads more like a defense attorney’s closing argument than an impartial investigation." It’s another version of
not a problem. The details are catalogued elsewhere [
why?…,
when?…,
why? again…], but the facts are pretty damning – particularly since they are focused on one of our most vocal spokespersons for psychiatry at large. So I presumptuously wrote the
Open Letter to the Board of Trustees and the Assembly of the American Psychiatric Association posted below.
While the Board of Trustees and the Assembly may look at the letter as detritus from the great unknown, I hope they take it seriously. The issue is much bigger than just the one on the table, and the reason for that is of their own making. We’ve literally had years of the APA minimizing, refuting, or ignoring what is now common knowledge – Conflicts of Interest have been an epidemic problem in psychiatry for years – at the top. This is no time for a terse internal blow off – another
not a problem. It is well beyond high time for the Trustees to take a stand that Conflicts of Interest will be taken as seriously as they should have been taken for years. And that the ethical standards for our leadership are the highest possible – not even the appearance of Conflicts of Interest. Our reputation has been indelibly tarnished already. It’s time for the Trustees to shed the damage control mentality of the past and turn this particular case over to an independent investigation, followed by an ethical renaissance that repositions us for the future. Dr. Lieberman began his presidency saying
seize the moment. This is the moment that is crying out to be seized…