by Antony Loebel, M.D. Josephine Cucchiaro, Ph.D. Robert Silva, Ph.D. Hans Kroger, M.P.H., M.S. Jay Hsu, Ph.D. Kaushik Sarma, M.D. Gary Sachs, M.D.American Journal of Psychiatry. 2014 171:160–168.
Objective: The authors evaluated the efficacy and safety of lurasidone in the treatment of patients with major depressive episodes associated with bipolar I disorder.Method: Patients were randomly assigned to receive double-blind treatment with lurasidone [20–60 mg/day [N=166] or 80–120 mg/day [N=169]] or placebo [N=170] for 6 weeks. Primary and key secondary endpoints were change from baseline to week 6 on the Montgomery-Åsberg Depression Rating Scale [MADRS] and depression severity score on the Clinical Global Impressions scale for use in bipolar illness [CGI-BP], respectively.Results: Lurasidone treatment significantly reduced mean MADRS total scores at week 6 for both the 20–60 mg/day group [-15.4; effect size=0.51] and the 80–120 mg/day group [-15.4; effect size=0.51] compared with placebo [-10.7]. Similarly, lurasidone treatment resulted in significantly greater endpoint reduction in CGI-BP depression severity scores for both the 20–60 mg/day group [-1.8; effect size=0.61] and the 80–120 mg/day group [-1.7; effect size=0.50] compared with placebo [-1.1]. Both lurasidone groups also experienced significant improvements compared with placebo in anxiety symptoms and in patient-reported measures of quality of life and functional impairment. Discontinuation rates due to adverse events were similar in the 20–60 mg/day [6.6%], 80–120 mg/day [5.9%], and placebo [6.5%] groups. The most frequent adverse events associated with lurasidone were nausea, headache, akathisia, and somnolence. Minimal changes in weight, lipids, and measures of glycemic control were observed with lurasidone.Conclusion: Monotherapy with lurasidone in t120 mg/day significantly reduced depressive symptoms in patients with bipolar I depression. Lurasidone was well tolerated, with few changes in weight or metabolic parameters.
by Antony Loebel, M.D. Josephine Cucchiaro, Ph.D. Robert Silva, Ph.D. Hans Kroger, M.P.H., M.S. Kaushik Sarma, M.D. Jane Xu, Ph.D. Joseph R. Calabrese, M.D.American Journal of Psychiatry. 2014 171:169–177.
Objective: Few studies have been reported that support the efficacy of adjunctive therapy for patients with bipolar I depression who have had an insufficient response to monotherapy with mood-stabilizing agents. The authors investigated the efficacy of lurasidone, a novel antipsychotic agent, as adjunctive therapy with lithium or valproate for the treatment of bipolar I depression.Method: Patients were randomly assigned to receive 6 weeks of double-blind adjunctive treatment with lurasidone [N=183] or placebo [N=165], added to therapeutic levels of either lithium or valproate. Primary and key secondary endpoints were change from baseline to week 6 on the Montgomery-Åsberg Depression Rating Scale [MADRS] and depression severity score on the Clinical Global Impressions scale for use in bipolar illness [CGI-BP], respectively.Results: Lurasidone treatment significantly reduced mean MADRS total score at week 6 compared with the placebo group [-17.1 versus -13.5; effect size=0.34]. Similarly, lurasidone treatment resulted in significantly greater endpoint reduction in CGI-BP depression severity scores compared with placebo [-1.96 versus -1.51; effect size=0.36] as well as significantly greater improvement in anxiety symptoms and in patient-reported measures of quality of life and functional impairment. Discontinuation rates due to adverse events were 6.0% and 7.9% in the lurasidone and placebo groups, respectively. Adverse events most frequently reported for lurasidone were nausea, somnolence, tremor, akathisia, and insomnia. Minimal changes in weight, lipids, and measures of glycemic control were observed during treatment with lurasidone.Conclusions: In patients with bipolar I depression, treatment with lurasidone adjunctive to lithium or valproate significantly improved depressive symptoms and was generally well tolerated.
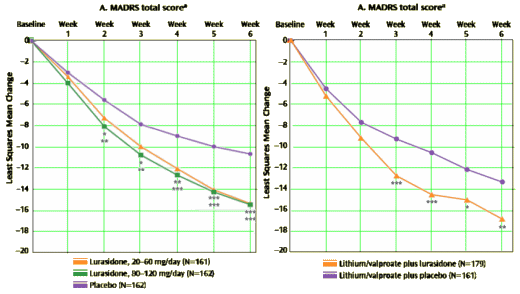
top study on the left, bottom study on the right
The two new articles are related to Lurasidone’s approval for Bipolar Depression. They report great efficacy and safety, but considering the disconnect between the published and the private versions last time around, I submitted an FOIA request for the approval documents [not linked on the Drugs@FDA web site]. Surprisingly, the Results Database on clinicaltrials.gov is completed. but has little information not found in the papers. I’m particularly interested to see how it fared in the US.
Editorialby Belmaker RHAmerican Journal of Psychiatry. 2014 171:131–133.Lurasidone is a new atypical antipsychotic developed by Sunovion Pharmaceuticals, Inc., that was approved for use in schizophrenia in the United States in 2010. It was approved for use in bipolar depression in July 2013, and the two back-to-back articles in this month’s Journal constitute pivotal studies for medication approval for bipolar depression by the FDA. Lurasidone is a strong dopamine D2 receptor blocker, as are almost all currently known antipsychotics used in schizophrenia, so its development and approval came as no surprise. Consistent with the major thrust of development in the schizophrenia treatment area today, lurasidone’s preclinical development was associated with the search for a dopamine D2 blocker with little or no extrapyramidal symptoms and little or no prolactin rise. Sometimes an absence of cholinergic muscarinic blockade and a felicitous balance of agonism and antagonism at some of the large number of serotonin receptor subtypes can achieve this desirable side effect profile. It has been achieved already in some other compounds, but the big prize today in pharmaceutical development of atypical antipsychotics is avoidance of the metabolic side effects such as weight gain, hyperlipidemia, and impaired glucose metabolism. The molecular basis of this cardiovascular profile is not fully known, so it has not been possible to use designer medicinal chemistry to create the ideal compound from basic principles. Some trial and error is necessary both in animals and eventually in clinical trials. Lurasidone may fit this basic bill…
Is this a new era in psychiatry and psychopharmacology? The history of psychopharmacology certainly saw some periods of discovery of entirely new principles, such as the first antipsychotic [chlorpromazine], the first antidepressant [imipramine], the first benzodiazepine, and lithium, the first mood stabilizer . On the other hand clinicians have become a bit jaded during a long era of “me too” compounds where new antipsychotics and new antidepressants seem to appear daily—hailed by leaders of the field and feted with dinners and weekends for clinicians willing to attend, later to lose their patents and be discarded on the scrap heap of history.
Lurasidone may be somewhere between those two situations. Sometimes incremental progress in psychopharmacology can gradually add up after much preclinical work, many clinical trials, and tinkering with several different compounds to an advance that is real. The discovery of a D2 blocker with a concomitant receptor profile that avoids extrapyramidal symptoms, avoids hyperprolactinemia, avoids cardiovascular side effects, and is also effective in bipolar disorder — this could be a serious advance of the field. Together with the large number of overlapping genetic linkages between schizophrenia and bipolar disorder, one might en- vision an impact on the battle over unitary psychosis theory that could even affect DSM-6.
What would a historian say? A historian might note that in the first edition of Diagnosis and Drug Treatment of Psychiatric Disorders by Klein and Davis, the first textbook of psychopharmacology, the usefulness of typical old-fashioned neuroleptics such as chlorpromazine in many forms of depression was emphasized and use of other typical neuroleptics for prophylaxis of bipolar disorder was widespread around the world, especially for patients who were non-adherent with lithium or in areas where blood testing was unavailable. It could be said that we have rediscovered the wheel. But it could be a better wheel than chlorpromazine or fluphenazine were in their times.
If you look as close as we can get to the Latuda® data in Schizophrenia, the data from outside the US is what got it approved [see ought to know by now…, in the shadows…]. So I hope the FDA FOIA will clear that up for these two large international studies in Bipolar Depression. But independent of the back story on the science, my emotional reaction was that I was reading some glossy magazine off the drugstore rack with advertisements for two new products – a screening test for depression and a new drug. It felt like it ought to have coupons like those pull-out sections of the Sunday paper. I worried that I was going off half cocked, and went back and read what I’d found out about Latuda® before, and lamented what I couldn’t find out about these two articles. But I couldn’t shake the feeling that the American Journal of Psychiatry was allowing itself to be a launching pad for new commercial products instead of the flagship journal for American scientific psychiatry. I didn’t know whether to be sad, or mad, or both…
“I didn’t know whether to be sad, or mad, or both…”
Are you at least a bit glad that in both these papers, there was only one academic psychiatrist co-author, as opposed to all those studies from last decade, which would have like a dozen academic psychiatrists from a dozen different institutions?
That’s one small step forward, right?
Surpise, surprise — Latuda, otherwise a loser in the atypical antipsychotic sweepstakes, turns out to be a psychopharmacological breakthrough for schizophrenia and “bipolar depression.”
Since “bipolar depression” is so very common in the general population, this news has generated a big primetime TV ad campaign and marketing outreach to physicians. Wouldn’t want to let a case of “bipolar depression” fall through the cracks, you know.
The takeover of the Am J Psychiatry by commercial interests is for the good of everybody.
Funny you should mention the glossy-magazine-advertisement bit. I was just reading People Magazine yesterday, and came across a two-page spread advertising Latuda for bipolar depression (featuring a picture of a nice-looking 20-something woman with a vaguely hopeful expression on her face).
Worse yet, the “safety information” had been carefully worded to repeatedly refer to Latuda as an “antidepressant.” People will be offered this drug for no better reason than “my antidepressant doesn’t seem to be working” — and will have no earthly idea what they are fooling around with.
Why was I reading People, you ask? Oh ,,, to see what they had to say about poor Philip Seymour Hoffman. They managed to get through several lurid, overwrought pages with only one offhand mention of “prescription drugs” although that is pretty clearly what brought Hoffman’s twenty years of sobriety crashing down around him.
Besides the two-page spread for Latuda, they also carried a two-page spread for lorcaserin (Belviq) another highly dubious quasi-antidepressant being flogged for weight loss. People Magazine can be hazardous to your health.
Some of us have actual subjective experience of taking these drugs with these different receptor affinities, and even going off of them. At times, I actually asked for gabapentin (Lyrica) since I had read some rave stuff about it in internet. Well, it didn’t do anything much good, but it didn’t do anything bad either. So, starting and stopping wasn’t a problem.
With Lurasidone, you just need to look at its receptor profile:
?1-adrenergic receptor (Ki = 48 nM)
?2A-adrenergic receptor (Ki = 1.6 nM)
?2C-adrenergic receptor (Ki = 10.8 nM)
D1 receptor (Ki = 262 nM)
D2 receptor (Ki = 1.7 nM)
5-HT2A receptor (Ki = 2.0 nM)
5-HT2C receptor (Ki = 415 nM)
5-HT7 receptor (Ki = 0.5 nM)
The same set of receptors all other neuroleptics work on. Many “atypical” neuroleptics work on histamine H1 histamine receptor which makes you sleepy and sedated. Well, the receptor will make you sleepy, you can confirm it by taking just 25 mg of Seroquel. I promise you’ll notice the effects. It may actually be one reason why Seroquel has become so popular, it gives people sleep.
Lurasidone acts on D2 just like all neuroleptics on higher doses, with all the adverse effects that come with that effect too. In some papers, they’ve made a point about the thing that it doesn’t affect H1, so it’s not that sedating. Haloperidol doesn’t affect H1 that much either, and with Seroquel the H1 effect has been advertised as a good thing. Lurasidone doesn’t have that much activity of H1 or muscarine, but it has plenty of activity on those serotonin receptors.
All of these drugs are playing with the same basic set of receptors, all of them block dopamine (D2) at “antipsychotic” doses. They have differences based on affinities on these receptors. This lurasidone works pretty similar to other drugs. Maybe the biggest difference to other atypicals is that it doesn’t affect so much histamine and muscarine, and therefore doesn’t have so many sedating and metabolic effects. But for instance, the D2 blocking and other receptor blocking is just as adverse or beneficial as with other neuroleptics. It just has a bit bit different profile on those same receptors. This stuff about how different drugs affect different receptors is the stuff Stahl, etc, are teaching about in those books and articles.
In short, lurasidone is basically only like an “atypical” antipsychotic without those sedating, etc, effects on histamine and muscarine receptors. The claim in that editorial in American Journal of Psychiatry simply claims that maybe this new drug has hit a “good” profile with these same set of receptors.
“The discovery of a D2 blocker with a concomitant receptor profile that avoids extrapyramidal symptoms, avoids hyperprolactinemia, avoids cardiovascular side effects, and is also effective in bipolar disorder — this could be a serious advance of the field.”
If they invented haloperidol this day, they could also promote that it doesn’t have the adverse metabolic effects of Zyprexa, etc.
I agree with Johanna.
I can remember when my doctors avoided prescribing a new drug because they wanted to see how the adverse effects shook out. But for some reason, that caution that seemed intrinsic to good medicine has gone by the wayside. Despite the discouraging evidence of the last 20 years, clinicians are always hot to try the latest psychiatric drug.
Is there something about the way medical students are chosen that selects for gullibility?
Alto,
I’m a trailing edge kind of guy, myself. I’ve really not seen this something new mania until the modern era. And it’s not just in psychiatry.
Thank you for your continued attention to this issue. My first thought when I saw this 2 studies in the AJP this month was “I wonder if ‘one boring old man’ will give the real story on this?”. Thanks!!!