I looked at the ClinicalTrials.gov Results Database several years ago, and decided it was an improvement over just having the published paper because of its strucyure, but was far short of having the raw data [see eyes wide shut open I…, eyes wide shut open II…, eyes wide shut open III…, and eyes wide shut open IV…]. But I have to say that it’s far more helpful to have it than not. And using it, you can get closer to knowing if you can trust the published article. So I’m all for Dr. Collins’ proposal to make it really non-optional, turning it into what it was intended to be [Honoring Our Promise: Clinical Trial Data Sharing and see promises, promises…].

I’m not going through the Results Section in detail for this study. It’s there for your perusal. In this case, there’s not much substantial differences between it and the paper. My contention would be that’s because it’s there. But look it over. It’s self explanatory. There’s nothing that makes all the site jockeying any clearer. Here’s the punch line as far as I’m concerned. First, from the paper:
Weight and vital signs. Mean baseline-to-endpoint weight change [LOCF] was significantly greater for OFCtreated patients [4.4 kg, standard error [SE]=0.2] than placebo-treated patients [0.5 kg, SE=0.3; p<.001]. Using MMRM methodology to model expected weight gain if all patients completed the 8-week study, mean weight change from baseline to week 8 was 5.1 kg [SE=0.3] for OFC-treated versus 0.6 kg [SE=0.4] for placebo-treated patients [p<.001]. Baseline-to-endpoint mean change in body mass index was also significantly greater for the OFC group [1.5 kg/m2, SE=0.1] than the placebo group [0.1 kg/m2, SE=0.1; p<.001]. Incidence of gaining ≥7% of baseline body weight was significantly greater for the OFC group relative to placebo [52% vs. 4%, p<.001], as was incidence of gaining ≥15% of baseline body weight [14% vs. 0%, p<.001]. No placebo patients and 2 OFC-treated patients gained ≥25% of their baseline.
Then from the ClinicalTrials.gov database:
I’m not sure I would want to prescribe a 10+ pound mean weight gain over two months in return for the statistical but not very large gain in efficacy.
And then there’s this, meaning they just made it under the wire with their guest author:
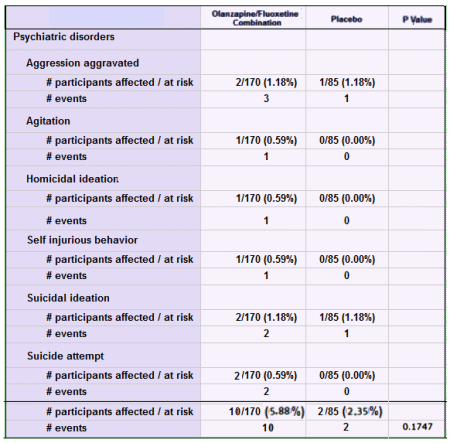
As I said, I’m not contesting this study, but the ClinicalTrials,gov Results Database keeps them honest. For example, it’s easy to check out something like the stats on total aggressive acts in the SAEs.
If we had the a priori Protocol, accurate information about the CRO that did the study, the true story about the sites used, the completed ClinicalTrials.gov Results Database, and the FDA NDA [New Drug Approval] Medical Report, we could actually do a decent job of vetting these Clinical Trial articles. But if we found something suspicious, we’d still need access to the raw data – Individualized Participant Data [IPDs] and perhaps the Client Report Forms [CRFs] – to actually run down any problems found.
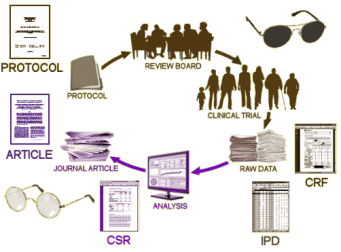
It sounds like a lot of hoops to make the trialists jump through, but it’s not. All of that information is already available. It would probably take them less time to put all that stuff up than it took me to write these three blogs. The pharmaceutical companies don’t want to do that because they want to be able to continue to accent the positive and eliminate the negative. And if they continue to act like they’ve acted in the last couple of decades, they’re going to lose the right to test their own products. Disease treatment is between the medical profession and our patients, not a playground for entrepreneurship and scientific deceit.
And as for
Symbiax®, who needs it? no matter what the trial says. It’s a patent extending experimercial to treat a questionable disorder. And even if you do use it, as Dr. Varner
pointed out, it costs $130/month more to take one pill a day instead of two. I’d like to thank Dr. DelBello and Eli Lilly for producing this fine example for my comments on Dr. Collins’ proposals. I hope that the JAACAP learns somewhere down the line that a ghost-written industry study with a guest author is still just an industry study, with all the conflict of interest problems that kept such studies out of our peer reviewed journals in our more scientifically honest past. Shame on Eli Lily and shame on the JAACAP…
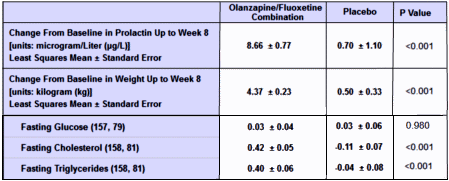

I have always found it interesting the dismissal of the weight loss issue. Almost any 15 year old or adult getting ready for the prom, or the beach, would find a 10+ pound weight gain to be depressing and only aggravate any self esteem issue.
The additional blood chemistry issues will elicit an immediate medication response resulting in a drug stew that will create its own drug interaction and stress. Given that the majority of anti-depressants are given by GP’s we will see additional drugs given as the effectiveness of the first drugs given diminish.
Cost is a real issue as many doctors have no idea what the cost is to their patients. They feel a co-pay is minimal when in fact multiple co-pays or excluded drugs can and do create a financial burden on families. As we watch Obamacare unravel we can see that costs are not declining, but doctors are facing new challenges in maintaining their practices. These challenges do include dealing with mental health issues.
Steve Lucas
The problem with writing early in the morning “weight loss” should be weight gain.
Steve Lucas
Disease treatment is between the medical profession and our patients, not a playground for entrepreneurship and scientific deceit.
YES!
This trial reported in JAACAP is the very essence of an experimercial – a drug trial designed and conducted to promote a product in a market niche – not to answer a meaningful scientific question. Shame on JAACAP, indeed, and shame on Lilly. Where is the meaningful discussion of Number Needed to Treat versus Number Needed to Harm? Where is a comparison of the combination product to either drug used alone for this population? Where is a comparison of this overpriced combo pill to simple and flexible use of the 2 inexpensive components? What prescriber really wants to be constrained by fixed dosages in the combination? How is that better for patients?
The first litigation I joined as a plaintiff’s expert, back in 1980, involved a woman with nonpsychotic depression who had been treated with one of the early fixed dose combinations of an antipsychotic (perphenazine) and an antidepressant (amitriptyline). She developed a disfiguring tardive dyskinesia and received over a million dollar judgment at trial. When the tragic cases start appearing with Symbyax we should wish more of the same on Lilly for this cynical marketing of an unnecessary product.
Dr. Carroll: I think there is a plausible, though paternalistic, rationale for the combination pill when treating depression in bipolar individuals. Because antipsychotics are aversive to many patients, when given 2 pills they tend to stop taking the antipsychotic and take only the antidepressant, and are then more likely to “switch” to a manic episode. I`ve seen it quite a few times.
Yes, Gad, that certainly is plausible.
Fixed combinations are stupid. I get the simplicity issue, but when you’re managing symptoms, you need the flexibility of dosage adjustments of each component.