Why all pharmaceutical research should be made open access
The government wants to make all publicly funded research available – but the same must be demanded of pharmas also
guardian.co.uk
by Cory Doctorow
20 November 2012… "All publicly funded pharma research," he said, as though correcting a mistake in elementary arithmetic. "If the public pays for it, they should see it, but if pharmaceutical companies want to pay for their own research, well …"
I knew where he was coming from. One of the strongest arguments for public access in scholarly and scientific publication is the "public debt" argument: if the public pays you to do research, the research should belong to the public. That’s a good argument, but it’s not the whole story. For one thing, it’s vulnerable to the "public-private partnership" counterargument, which goes, "Ah, yes, but why not ensure that the public gets a maximum dividend on its spending by charging lots of money for access to publicly funded research and returning the profit to the research sector?" I think this argument is rubbish, as do most economists who have studied the question…
That’s why Goldacre’s work is important to this discussion. The reason pharma companies should be required to publish their results isn’t that they’ve received a public subsidy for the research. Rather, it is because they are asking for a governmental certification saying that their products are fit for consumption, and they are asking for regulatory space to allow doctors to write prescriptions for those products. We need them to disclose their research – even if doing so undermines their profits – because without that research, we can’t know if their products are fit for use…
And that’s why big pharma needs to show its work: because regardless of their bottom line, their products mustn’t be allowed into the market without such a showing. It’s important to get publicly funded work into the public’s hands, but that’s where the open access story starts, not where it ends.
There’s a successful movement in the computer world – Open Source. It means when you purchase a piece of software, you get the code too. You can alter it if you want. The Linux operating system is Open Source, and provides the kernal for lots of things – like Android, or the Apache Server that sent you the page your reading right now. If you’re using the FireFox Browser like I am, you’re using Open Source. The only reason I’m on a Windows computer is that I do a lot of GIS [mapping] and the mapping software isn’t available for Linux. I guess the other reason is that the graphics programs for Linux are hard to use. Why use Open Source? A lot of it is free, but that’s not why I use it whenever I can. I use it because it’s right. Even though I have only modified a few programs myself, if I wanted to look at the code, I could. It’s just an honesty thing.
The data from Clinical Trials is the same way. Very few people either care to or have the expertise to review the Data and mathematical transformations involved, but there are some that can – and that keeps people honest. All we can do with the published studies is check what we can see. Sometimes we can find things, as in this table from the Gibbons papers about antidepressants that is riddled with errors:
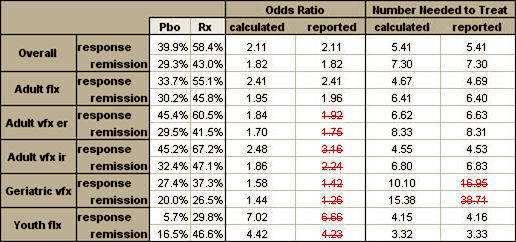
But with the open access, we can vet the raw data in Paxil Study 329 [showing its non-significance] using only an Excel Spreadsheet:
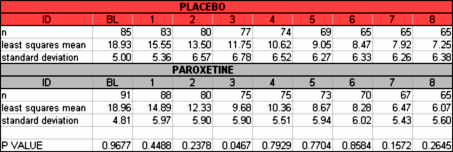
or see that the reason they changed the primary outcome variable was to capitalize on an outlier value [circled] that really didn’t characterize the data:
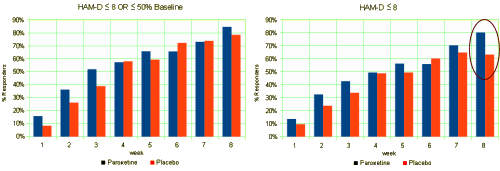
or look at the suicidality data and see the true story:
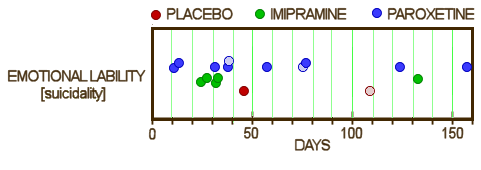
And if an old non-biological psychiatrist can see this much, imagine what a trained neuroscientist or statistician can see. Not many people will crunch someone else’s numbers, but some will, and that’s a powerful force.
Make no mistake, Open Access to Clinical Trial raw data will have a negative impact on the Clinical Trial Industry and severely restrict the Psychotropic Drug industry. It will mean that there will be a whole lot fewer drugs on the market and it will radically alter the life styles of the Key Opinion Leaders that have thrived for twenty-five years on the bonanza. The crisis of financing psychiatry departments we had in the 1970s will return. The managed care mavens who have supported drug treatment as cost effective alternative will balk. And so the inertia of data secrecy isn’t just supported by habit or tradition or even some natural force like gravity. It’s actively maintained because it has been so incredibly lucrative to a whole lot of people. Changing direction is going to be a hard task.
Of interest:
http://neuroskeptic.blogspot.com/2012/11/the-perils-of-sharing-brain-scans.html?m=1
http://web.archive.org/web/20020720024810/http://www.psy.vanderbilt.edu/faculty/gauthier/fmridc_letter.html
http://web.archive.org/web/20010629100631/http://www.nas.edu/includes/letter.htm
At the top:
“Excerpt from a letter by the President of the National Academy of Sciences.
“Permitting the researcher who actually collected the data to be the first to analyze and publish conclusions concerning the data is an essential motivational aspect of research. “”
Below:
“We are particularly concerned with any journal’s decision to require all authors of all fMRI related papers accepted for publication to submit all experimental data pertaining to their paper to the Data Center. Mandatory submission of fMRI data impinges on the rights authors should have on the publication of findings stemming from their own work. In particular, a paper can often test a hypothesis about a small region of the brain or a subset of the manipulations that were performed during the experiment. The nature of fMRI data is such that it may be difficult to separate the data that is relevant to a published paper from data that is destined to another manuscript. Research ethic obligates us to provide anyone interested the data that is relevant to a particular paper and we certainly intend to abide by this convention. When such requests are made directly between scientists, it is easy to inform one another of the data that is relevant to published claims as opposed to the data we wish to remain in our possession for future use. The requirement to make an entire data set public will make this very difficult, if not impossible. Augmenting our concern is the fact that neither the letter announcing the creation of the center nor the current web site for the project (www.fmridc.org) make any mention of how these issues may be addressed (for example, a time delay on public availability of data or an option for researchers not to submit their data if they hope to publish a paper using a different subset of the data at a later point). Moreover, even if these issues were addressed, making such data publicly available might raise issues regarding copyrights and the prior release of submitted work.”
Multi-site trials clinical trials (especially those that are NOT pharma funded) are driven by the idea that the enormous amount of time and effort (not to mention money) that went into them results in multiple papers that fuel the academic careers of the participants. NIMH and other funding sources as well as academic centers fuel this culture. Ignoring those realities and simply saying that ethics demand a different path seems similar to encountering a dysfunctional family and dispensing advice so far from their capabilities as to be effectively useless. Scientists are bred to be protective of their data.
I haven’t read the 1999 letter but suspect that that earlier debate is also worth reviewing.
The publications from multi-site trials go on for years. Witness the NiMH trials published during the last decade. I hope you have a chance to review the 1999 letter. If these open data proposals are going to meet a buzz saw among the vast majority of researchers that is a concern regarding their viability. At that point the main thing accomplished would be advancing the academic prestige of those forwarding the argument: e.g., Goldacre.
Does the open data movement have protections built in that limit the publication of original findings to the original authors? We apply copyright/usage protections to pretty much all fields I human endeavor. I chances here is a chance to introduce more transparency, but if people are hoping to revolutionize science beyond that, to something with little to no parallel elsewhere (which making all the data available without first use protection would do) then it will be doomed.
I have not read the BMJ editorial (my bad). If this issue is addressed then my apologies. Given that this is likely the first concern that would be raised by most academic researchers (it has long been pointed out that the biosciences do not have a physics model) it might be worth explicitly addressing this concern up front.
I truly applaud Goldacre for his efforts, but they might be diminished were he to not accrue financial and academic capital for his efforts.
I don’t want to see the transparency movement killed by disinformation. Or, by sister petitions and leters paralleling the ones referenced above.
Arguments can be made for a more radical approach:
http://online.wsj.com/article/SB10001424052970204644504576653573191370088.html?mod=googlenews_wsj
However, I don’t want to have to wait for a readiness for that seismic shift in order to see improvement on the transparency front. And, to see that siesmic shift will take action by the NIMH and academic centers.
How has waiting for that been working for us?
As the Scientific American article argues the most immediate opportunity lies with professional societies and journals.
The transparency movement needs the bulk of researches as allies, or at least not to be opponents.
I think transparency can, perhaps, survive some of the fears it will engender in researchers. I doubt it can survive the fear of loss of right to first publication. The fear articulated in those letters/petitions above. I don’t know of a single bioscience researcher who would not oppose something that would limit their privileged status re their own data to just one publication. I can imagine several who would support these transparency efforts if that concern is addressed. It may be in the BMJ proposal, but the reporting of it has not made that clear.