An Editorialby H. Christian FibigerSchizophrenia Bulletin. 2012 38[4]:649–650.
Psychopharmacology is in crisis. The data are in, and it is clear that a massive experiment has failed: despite decades of research and billions of dollars invested, not a single mechanistically novel drug has reached the psychiatric market in more than 30 years. Indeed, despite enormous effort, the field has not been able to escape the “me too/me [questionably] better” straightjacket. In recent years, the appreciation of this reality has had profound consequences for innovation in psychopharmacology because nearly every major pharmaceutical company has either reduced greatly or abandoned research and development of mechanistically novel psychiatric drugs. This decision is understandable because pharmaceutical and biotechnology executives see less risky opportunities in other therapeutic areas, cancer and immunology being the current pipeline favorites. Indeed, in retrospect, one can wonder why it took so long for industry to abandon psychiatry therapeutics. So how did we get here and more importantly, what do we need to do to find a way forward?…
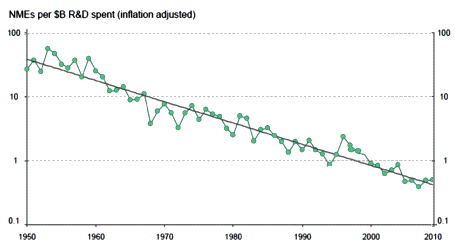
Click the graphic for the article
I wrote "an honest assessment of the current era" when I read Dr. Fibiger’s article in 2012 and "a wise voice" when I reread it this week. I want to clarify that it’s that paragraph up there [minus the last sentence] that I was talking about. In what follows, he gets into "find a way forward" too quickly when what we need to figure out is "how did we get here." In my opinion, his problem with sustained wisdom is in "more importantly," revealing that you can take the boy out of industry but you can’t take the industry out of the boy may be an operative principle clouding his subsequent wisdom. What’s important to me [and us] is where in the hell have we been?
As much as I’m obviously in love with that graph, there’s something important that it doesn’t show – Clinical Trials. Clinical Trials proving efficacy [lite] were added to the mix in 1962 as a reform measure – including efficacy with safety in the FDA’s task list for approval of a drug. The Clinical Trial Industry blossomed later – 1980s? While there are many things one can say about that change, the one I’m focused on right now is the ability of a Clinical Trial to detect much smaller differences – even clinically insignificant differences. We don’t need Clinical Trials for Morphine [antiquity] or Penicillin [1928] or Thorazine [1950] or Valium [1960]. They obviously have strong effects – no statistics required. But for many medications, the math is necessary.
Finally, a personal observation of my own. The pharmaceutical industry in the 1960s and early 1970s that I met as a young Internist and the contemporary pharmaceutical industry I now write about seem like different species to me. I’ve never been personally much involved with industry, but from my perspective, the deceitful practices I write about here were not there at an earlier time. And in the interim, I was in a situation where I had little interest or contact, so I can’t really comment on the process of change. I can only say that the change seems striking to me.
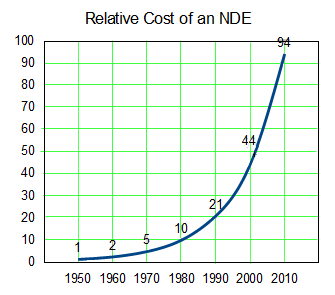
This is the trend line from the top graph expressed in a different way. To get an NDE to take to market costs me 94 times as much in contemporary dollars in 2010 as it cost me in 1950. And that 2010 NDE is backed only by statistical evidence from a Clinical Trial rather than evidence that can be seen from across the room as in 1950 – so my lackluster 2010 drug that it costs me 94 times as much to get hold of is up against a robust 1950 drug [and, by the way, they’re not nearly so many of those NDEs around any more – except maybe out of reach in the top of the tree].
I am not entirely comfortable with this answer as it doesn’t explain everything, but…
Clinical trials and their statistics being controlled by the producers/sellers without a means for buyers to verify it.
With the data in their hands it became more profitable in the near term to produce clinical trial results from tweaking existing chemistries and statistics than to develop novel medications. Money was diverted to this instead of being used to further research.
There is a concept I call the Golden Child. Children born between 1940 and 1950 were golden. A country having been battered by a depression and a world war were looking for any sign of hope, and in these children they saw a bright future.
Little Johnny would steal cookies before dinner and be given a glass of milk as this was viewed as a prank. Later we would not let little Susie ruin her life with an unwanted pregnancy, so abortion became the law of the land.
These children’s parents having fought in WWII went to college on the GI Bill and some never left, staying to teach. They brought with them all of the poverty of their youth and the belief that big government was the solution, just as it was in their youth.
Starting around 1980 this greatest generation started leaving the work force and with them a sense of fair play. Polishing the apple had been acceptable, lying was not. Worrying about your customer was pushed aside in favor of finding new customers if one failed.
Johnny and Susie took over and relied on their experience of always getting their way, climbing the corporate ladder with always rising salaries and larger staffs. Staffs can become a shield against failure as they take the blame for problems and no one person is held accountable.
Johnny and Susie were use to the lie and there were no limits to what they would do to get their way. Managers who in the past would have stopped their corporate rise were pushed out of the way in the never ending goal of improved quarterly numbers, and Johnny and Susie fit right in.
Today pharma’s corporate culture is a product of the Golden Child. Self centered, interested in short term gain, and willing to do anything to achieve their goals. Their college instructors taught them how to use the system and argue opposing views, and in the words of one philosopher: there are no facts, only interpretations.
Our government is filled with the remnants of this group and their children. Big government is good and we need to study all aspects of every problem because those terrible corporations should not make money, only government creates jobs and businesses. Pharma with its ties to academia, the NIH, and other government agencies is more than willing to play the political game and can use politics and the legal system for cover.
When I started grad school an instructor told us that business is always living 25 years in the past. Those who have suffered the repeated economic down turns and political doubletalk of the recent past have yet to rise to positions of authority. Johnny and Susie are on the way out.
This younger generation has spent a life time of being hyped. Watched their parent’s loose jobs due to poor political decisions, are struggling with massive student debt, and have a very different view than Johnny and Susie.
They understand that facts are the enemy of truth.
Steve Lucas
I don’t know that the young think so differently than their parents. I work closely with a dozen 20-somethings and they’re pretty much all in the government is good camp.
And most are into climbing the corporate ladder whether by hard work, kissing-up, self-promotion or discreetly stabbing someone in the back. Generally some combination of all of these.
I tend to be patient with them as it’s fairly obvious that’s all they know, and their behavior is no where near what I saw when I worked in banking.
Because more and more candidate drugs are washing out during the NDEs:
http://www.nature.com/nrd/journal/v8/n12/abs/nrd2961.html
Nature Reviews Drug Discovery 8, 959-968 (December 2009)
Bernard Munos
Lessons from 60 years of pharmaceutical innovation
“Despite unprecedented investment in pharmaceutical research and development (R&D), the number of new drugs approved by the US Food and Drug Administration (FDA) remains low. To help understand this conundrum, this article investigates the record of pharmaceutical innovation by analysing data on the companies that introduced the ~1,200 new drugs that have been approved by the FDA since 1950. This analysis shows that the new-drug output from pharmaceutical companies in this period has essentially been constant, and remains so despite the attempts to increase it. This suggests that, contrary to common perception, the new-drug output is not depressed, but may simply reflect the limitations of the current R&D model. The implications of these findings and options to achieve sustainability for the pharmaceutical industry are discussed.”
Full PDF text: Google
munos researchgate lessons
The PDF is at the top of results.
The discovery of truly new drugs relies on the discovery of truly new pathophysiological explanations for illness and new (and more valid) behavioral tests for drug efficacy. Because this type of research is more speculative, it is more tempting to exploit existing knowledge and find more “me too” drugs.
Still, there are some very interesting unexploited findings in the antidepressant effects of ketamine and similar drugs and some novel peptide neurotransmitters associated with psychosis. These might eventually open up new avenues of pharmacotherapy, or they may be dead ends. If they are successful, however, expect other companies to start the “me too” research right away.
NDMA antagonism is a novel approach but probably two decades away. Ketamine seems to work for about 2 weeks, but as far as office use, it’s not ready for prime time. But at least they’re trying something different.
Merck’s new orexin antagonist sleeper was approved but I understand it’s going to be priced at 10 bucks a pill. I don’t get it. How is that possibly going to compete with existing products? A lot of these new drugs are pricing themselves out of any formulary. I hope this isn’t a pattern, but I see a lot of new drugs are doing this. I don’t get it even from a greed/marketing standpoint especially now that Lunesta is off patent. No one is going to come to your restaurant if you charge 100 bucks for a hamburger no matter how good it is.
Unfortunately, Doctor O’Brien, you’re wrong.
http://www.thrillist.com/eat/nation/most-expensive-burgers
Which brings up insurance companies and TMAP— why were insurance companies willing to pay for so many high dollar medications that were still on patent? Of course, part of the deal was ripping off MEDICAID, but insurance companies often do pay too much.
Wiley,
Now those are some burgers!
Nice find. I’ve naively underestimated the stupidity of the 1st percentile consumer, a mistake I still make on occasion though I should know better. I guess now that we’ve identified a 100 dollar burger, I think it’s safe to say that Five Guys and and In and Out won’t be selling them anytime soon. Which is to say that I can’t see any formulary or Medicare agreeing to a 10 dollar sleeping pill.
I don’t get 1.5 billion dollars and trying to compete at that price point.
Forbes agrees:
http://www.forbes.com/sites/matthewherper/2012/10/31/can-merck-wake-the-sleeping-pill-market-it-depends-on-ambiens-side-effects/
Generic Lunesta makes them dead on arrival IMHO
top 1 percent, typo, sorry
I think they are going for the $10/tab price point because that’s what Silenor is going for, and that appears to be on the formulary of several Medicare plans even though it amounts to repackaged doxepin.
Regardless, I have found health insurance companies to be as penny-wise and pound-foolish as any other company I’ve dealt with. I don’t see them as fiduciary bodies in any way, shape or form.
They’ll pay these prices while raising co-pays on their high volume generics – until they can’t anymore. And, maybe, for now they are still into selling the myth of the progress by having a few new brand names around.
The price is a Debbie Downer on my enthusiasm for the concept. Which is, suppress wakefulness instead of pushing GABA. But I’m reading that there still are issues with next day drowsiness, so when all is said and done, what’s the advantage?
Can’t speak to the advantages or disadvantages, but, I guess this new sleeper may work very well for some people. And, knowing how sleep deprivation really is a form of torture, I know they’d being willing to pay for it.
I agree, the price is a downer. The only good thing to say is that it’s about half the cost of wakefulness. Check out what Modafinil goes for.
It is my understanding that many sleep meds like Lunesta and Ambien don’t address sleep maintenance insomnia like the new Merck Drug allegedly does. And if folks have had bad experiences trying ADs for sleep maintenance insomnia, they might be willing to pay the steep price to try this med since as Arby mentioned, sleep deprivation is h-ll.
In response to Dr. Mickey’s original question: It could be the paradigm of treatment that’s held administration of toxins to counter disease processes, which is only about 200 years old, is applicable only in limited situations.
As we can see, in antibiotics, the first big NMEs, the paradigm is just about worn out as nature exerts an inevitable homeostasis via bacterial mutation.
The business cycle of pharmaceutical companies following this paradigm illustrates this. It’s getting more and more expensive to find effective toxins.