Nosing around about Ketamine, I ran across this draft that I never posted from 2½ years ago:
from February 19, 2014: Neuroskeptic has an interesting thing going trying to figure out if the effect on depressed people from Ketamine is because Ketamine is a pharmacologic antidepressant, or, to put it in my own worlds, does getting stoned ripped dissociated feeling something psychodelic help depressed people? He starts by discounting studies that compare Ketamine to Placebo since Ketamine is obviously psychoactive and a Placebo isn’t. Then he moves to a study that compared Ketamine to a Benzodiazepine – another psychoactive drug. That’s difficult because though Benzodiazepines are psychoactive, they’re sedatives. Ketamine is something else. He mentions a study that’s not in depressed people, but it’s pretty interesting. The subjects were Cocaine addicts who wanted to quit. They compared Ketamine to a benzodiazepine too. Here are the results of the questions they asked:
 That’s going to be a hard comparator to find! And it begs the question if it’s helping because it gets the depressed "out of themselves." If you’ve worked an ER where people have been brought having taken Ketamine, it’s just hard to imagine using it as a therapeutic agent. They are way "out of themselves!" So here’s what Neuroskeptic said:
 In summary I think the question is still very much open whether ketamine’s antidepressant effects are ‘psychological’ or ‘physiological’ in origin. I’m genuinely agnostic on the issue; I would love to know the answer, but at present we don’t have it. In summary I think the question is still very much open whether ketamine’s antidepressant effects are ‘psychological’ or ‘physiological’ in origin. I’m genuinely agnostic on the issue; I would love to know the answer, but at present we don’t have it.
I’m going to vote ‘psychological’…
|
I’m obviously not quite so agnostic as Neuroskeptic when it comes to Ketamine, but I’m open to being born·again given something really solid. The question remains, "Is the manifest improvement in depressive symptoms following the initial psychodelic experience ‘psychological’ or ‘physiological’?" and I have to concede that I don’t really know the answer. I certainly can’t argue that there’s not an effect [this Janssen article from the recent AJP]:
by Jaskaran B. Singh, Maggie Fedgchin, Ella J. Daly, Peter De Boer, Kimberly Cooper, Pilar Lim, Christine Pinter, James W. Murrough, Gerard Sanacora, Richard C. Shelton, Benji Kurian, Andrew Winokur, Maurizio Fava, Husseini Manji, Wayne C. Drevets, and Luc Van Nueten
«Janssen Employees, Janssen COI»
American Journal of Psychiatry. 2016 173:816–826.
Objective: Ketamine, an N-methyl-D-aspartate glutamate receptor antagonist, has demonstrated a rapid-onset antidepressant effect in patients with treatment-resistant depression. This study evaluated the efficacy of twice- and thrice-weekly intravenous administration of ketamine in sustaining initial antidepressant effects in patients with treatment-resistant depression.
Method: In a multicenter, double-blind study, adults [ages 18–64 years] with treatment-resistant depression were randomized to receive either intravenous ketamine [0.5 mg/kg of body weight] or intravenous placebo, administered over 40 minutes, either two or three times weekly, for up to 4 weeks. Patients who discontinued double-blind treatment after at least 2 weeks for lack of efficacy could enter an optional 2-week open-label phase to receive ketamine with the same frequency as in the double-blind phase. The primary outcome measure was change from baseline to day 15 in total score on the Montgomery-Åsberg Depression Rating Scale [MADRS].
Results: In total, 67 [45 women] of 68 randomized patients received treatment. In the twice-weekly dosing groups, the mean change in MADRS score at day 15 was -18.4 [SD=12.0] for ketamine and -5.7 [SD=10.2] for placebo; in the thrice-weekly groups, it was -17.7 [SD=7.3] for ketamine and -3.1 [SD=5.7] for placebo. Similar observations were noted for ketamine during the open-label phase [twice-weekly, -12.2 [SD=12.8] on day 4; thrice-weekly, -14.0 [SD=12.5] on day 5]. Both regimens were generally well tolerated. Headache, anxiety, dissociation, nausea, and dizziness were the most common [>20%] treatment-emergent adverse events. Dissociative symptoms occurred transiently and attenuated with repeated dosing.
Conclusions: Twice-weekly and thrice-weekly administration of ketamine at 0.5 mg/kg similarly maintained antidepressant efficacy over 15 days.
In May, an article published in Nature got a lot of attention. It suggested that the psychodelic effects of Ketamine and the antidepressant effects came from different molecules. Great news, but the problem was that the finding was studied in mice, not people:
by Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr, Gould TD
Nature. 2016 533[7604]:481-486.

Major depressive disorder affects around 16 per cent of the world population at some point in their lives. Despite the availability of numerous monoaminergic-based antidepressants, most patients require several weeks, if not months, to respond to these treatments, and many patients never attain sustained remission of their symptoms. The non-competitive, glutamatergic NMDAR [N-methyl-d-aspartate receptor] antagonist [R,S]-ketamine exerts rapid and sustained antidepressant effects after a single dose in patients with depression, but its use is associated with undesirable side effects. Here we show that the metabolism of [R,S]-ketamine to [2S,6S;2R,6R]-hydroxynorketamine [HNK] is essential for its antidepressant effects, and that the [2R,6R]-HNK enantiomer exerts behavioural, electroencephalographic, electrophysiological and cellular antidepressant-related actions in mice. These antidepressant actions are independent of NMDAR inhibition but involve early and sustained activation of AMPARs [α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors]. We also establish that [2R,6R]-HNK lacks ketamine-related side effects. Our data implicate a novel mechanism underlying the antidepressant properties of [R,S]-ketamine and have relevance for the development of next-generation, rapid-acting antidepressants.
Discover Magazine
By Neuroskeptic
May 7, 2016
In recent years there has been great research interest in ketamine as an antidepressant. Ketamine, a drug better-known for its use as an anaesthetic [and a recreational drug in lower doses] is claimed to have powerful, rapid-acting antidepressant effects, even in depressed patients who have not responded to more conventional drugs. However, its mechanism of action remains unclear.
Now, in a major new Nature paper, Baltimore researchers Panos Zanos and colleagues say that ketamine itself is probably not an antidepressant after all. Instead, the antidepressant effects can be attributed to a metabolite of the drug, formed in the body after taking ketamine. The metabolite is called [2S,6S;2R,6R]-HNK. But is this a real breakthrough or a red herring? Here’s how Zanos et al. describe their findings:
Here we show that the metabolism of [R,S]-ketamine to [2S,6S;2R,6R]-hydroxynorketamine [HNK] is essential for its antidepressant effects, and that the [2R,6R]-HNK enantiomer exerts behavioural, electroencephalo-graphic, electrophysiological and cellular antidepressant-related actions in mice.
So how does HNK do this? Unlike ketamine, HNK is not an NMDA glutamate receptor antagonist. Rather, it acts to promote signalling via the AMPA glutamate receptor. NMDA antagonism is believed to underlie ketamine’s anaesthetic and psychoactive [dissociative hallucinogenic] properties. So HNK is not likely to have these undesirable effects, Zanos et al. say, making it more suitable as an antidepressant.
I’ve been following the ketamine/depression story for nearly a decade and this paper is the most interesting piece of work I’ve seen. This is a really impressive set of experiments – but they were all in animals. Zanos et al. haven’t shown that ketamine’s antidepressant effects are mediated by HNK in people; rather they’ve studied the ‘antidepressant-like‘ effects of ketamine in rodent behavioural models such as the forced swim test. Bottom line: we don’t yet know whether HNK is a human antidepressant.

 My own suspicion is that the antidepressant effects of ketamine may be driven not by a metabolite but as a kind of psychological reaction to its pronounced psychoactive effects. If HNK is non-psychoactive, I predict that it won’t turn out to mediate ketamine’s antidepressant effects in humans. HNK might nonetheless have some antidepressant activity but not as dramatic as ketamine. Then again I might very well be wrong, and I hope I am, because if Zanos et al. are right, they might just have discovered the next ‘miracle’ antidepressant.
My own suspicion is that the antidepressant effects of ketamine may be driven not by a metabolite but as a kind of psychological reaction to its pronounced psychoactive effects. If HNK is non-psychoactive, I predict that it won’t turn out to mediate ketamine’s antidepressant effects in humans. HNK might nonetheless have some antidepressant activity but not as dramatic as ketamine. Then again I might very well be wrong, and I hope I am, because if Zanos et al. are right, they might just have discovered the next ‘miracle’ antidepressant.
Here are a couple of other commentaries on the HNK finding:
For ever-so-long, we’ve lived in a dream world in psychopharmacology. I call it future-think, what’s coming down the
pipeline or shows promise. But the pipeline is dry, and HNK, mentioned above, hasn’t even made it into humans yet. This story has one of the classic signs of future-think, a review article from Dr. Charlie Nemeroff, KOL extrordinaire. In
Ketamine and Other NMDA Antagonists: Early Clinical Trials and Possible Mechanisms in Depression, he was pushing
Rapastenel, touted to be a Ketamine-ish drug with antidepressant properties but none of the club drug effects [see
a touch of paralysis…]. Nemeroff earlier went out of his way to assure us he had no COI with the drug. However, right after the review article came out, the drug and its company was bought up by
Allergan, one of Dr. Nemeroff’s COI companies [
After a $560M Allergan buyout, Naurex vets regroup for CNS R&D] [up to his old tricks?]. It’s based on a single
proof of concept study [
Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent], marginal if even that, but somehow Allergan got it on the fast track [
Allergan’s Rapastinel Receives FDA Breakthrough Therapy Designation for Adjunctive Treatment of Major Depressive Disorder [MDD]]. They advertize Phase III trials in the offing, but none are registered on clinicaltrials.gov that I can locate.
So I wouldn’t say Rapastenel is either in the pipeline or yet even a drug of promise, but it’s being discussed as if it is the next great something-or-another.And Janssen is certainly going forward full bore with Esketamine intravenous and intranasal [see the best predictor… and Esketamine Receives Breakthrough Therapy Designation from U.S. Food and Drug Administration for Major Depressive Disorder with Imminent Risk for Suicide]. When I looked at their recent study earlier [Intravenous Esketamine in Adult Treatment-Resistant Depression: A Double-Blind, Double-Randomization, Placebo-Controlled Study], I focused on efficacy and didn’t pay enough attention to the club drug effect. They assessed it with both the Brief Psychiatric Rating Scale [BPRS] total score and the Clinician Administered Dissociative States Scale [CADSS] total score [which roughly paralleled each other]. The upper figure is the BPRS data from the paper and the lower versions are redrawn with an accurate x-axis timeline [because I am suspicious of irregular scales in graphic presentations]. It looks like about a 2 – 4 hour "high":
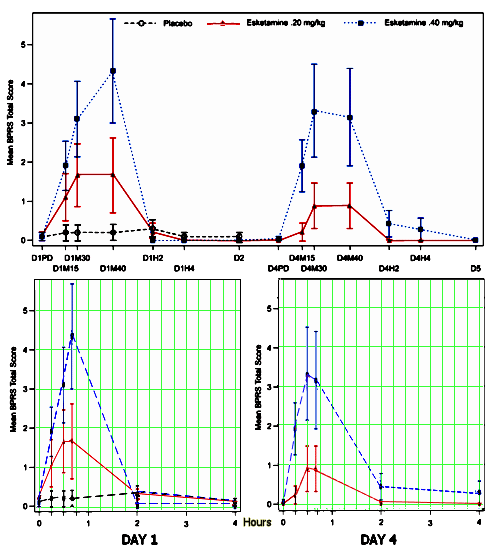
"Similar to ketamine, esketamine led to transient dissociative and psychotic symptoms. According to CADSS severity categories, the peak mean CADSS total scores at 40 minutes postinfusion on day 1 for the esketamine .20 mg/kg and .40 mg/kg groups would be categorized as high. However, symptoms subsided to baseline levels within 4 hours. The CADSS scores showed evidence of dose dependence on each DB dosing day. A similar pattern was observed for psychotic-like effects measured using BPRS total scores. These results are similar to results of IV ketamine studies."
The intranasal form [study not yet published that I can find – reference: Canuso C, et al. "Esketamine for the Rapid Reduction of the Symptoms of Major Depressive Disorder, Including Suicidal Ideation, in Subjects Assessed to be at Imminent Risk for Suicide." Society of Biological Psychiatry 71st Annual Scientific Meeting. May 12-14, 2016] may be an improvement in terms of a delivery system, but it’s not apparent to me how Esketamine makes any advances over the already available racemic mixture of Ketamine in terms of either efficacy or absence of club drug effects.
I am perhaps even more skeptical than Neuroskeptic that the transient antidepressant effect that follows the psychodelic effect of these drugs can be isolated as the "the next ‘miracle’ antidepressant" – agreeing with him that the effect is likely a linked consequence of the drug’s mind-altering experience. But beyond that, I think of Ketamine as a feel good drug rather than an antidepressant, adding a good feeling instead of ameliorating the target emotion – depression. I question whether this is a rational direction for psychiatric treatment to pursue.
 [click image for the SSRis, SNRIs, and Atypicals by the year of Approval]
[click image for the SSRis, SNRIs, and Atypicals by the year of Approval]
While the debates about the psychoactive drugs that have flowed from the pharmaceutical pipeline [see
the pipeline paradigm…] have covered lots of territory over the last thirty-five years, at least we haven’t had to deal so much with the problems of an earlier generation – drug abuse [Benzodiazepines, Barbituates, Meprobamate, etc.]. Ketamine adds that back into the equation. It is already a widely abused street drug, and there’s no reason to doubt that its use as an antidepressant will dramatically expand its abuse potential – particularly if there’s
an intranasal a snortable version. It may be consistent with the goals of the pharmaceutical industry [sell more drugs] or managed care [cut hospitalization costs], but this feels like
no country for psychiatry [or our patients] – like a time to be careful rather than in a rush. So I question the FDA fast-tracking either
Rapastinel or
Esketamine by granting
Breakthrough Therapy Designation. If a non-psychodelic antidepressant actually comes from this line of thinking down the road, then that might be reasonable. But for the moment, Ketamine’s cousins remain inhabitants of the realm of speculation and entrepreneurialism, hardly candidates for the fast lane…


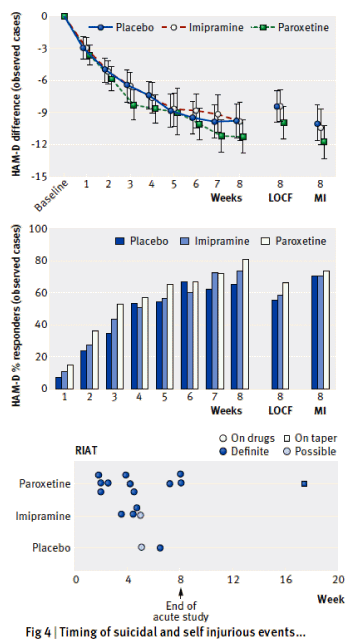

 ure enough, six months later I got hold of The Rothman Report [see
ure enough, six months later I got hold of The Rothman Report [see  As much as we’d like our antidepressants to help this much or this often, they’re more in this range, and it may take several weeks before you notice any change."
As much as we’d like our antidepressants to help this much or this often, they’re more in this range, and it may take several weeks before you notice any change." Unless you’re a race car driver with a manual transmission, you don’t notice the dashboard gauge on the far right. You just glance at the speedometer on the left that tells you how fast you’re going [mph]. The tachometer on the right tells you how fast the motor’s turning [rpm]. It lets the speed demon know to shift before it gets "in the red" or the motor will self destruct.
Unless you’re a race car driver with a manual transmission, you don’t notice the dashboard gauge on the far right. You just glance at the speedometer on the left that tells you how fast you’re going [mph]. The tachometer on the right tells you how fast the motor’s turning [rpm]. It lets the speed demon know to shift before it gets "in the red" or the motor will self destruct. Well Ben Goldacre isn’t still 12 years old, but he’s certainly in the generation that can see the possibilities. And he’s hooked up with the
Well Ben Goldacre isn’t still 12 years old, but he’s certainly in the generation that can see the possibilities. And he’s hooked up with the 
 In summary I think the question is still very much open whether ketamine’s antidepressant effects are ‘psychological’ or ‘physiological’ in origin. I’m genuinely agnostic on the issue; I would love to know the answer, but at present we don’t have it.
In summary I think the question is still very much open whether ketamine’s antidepressant effects are ‘psychological’ or ‘physiological’ in origin. I’m genuinely agnostic on the issue; I would love to know the answer, but at present we don’t have it.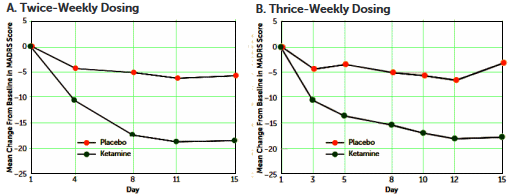
 Major depressive disorder affects around 16 per cent of the world population at some point in their lives. Despite the availability of numerous monoaminergic-based antidepressants, most patients require several weeks, if not months, to respond to these treatments, and many patients never attain sustained remission of their symptoms. The non-competitive, glutamatergic NMDAR [N-methyl-d-aspartate receptor] antagonist [R,S]-ketamine exerts rapid and sustained antidepressant effects after a single dose in patients with depression, but its use is associated with undesirable side effects. Here we show that the metabolism of [R,S]-ketamine to [2S,6S;2R,6R]-hydroxynorketamine [HNK] is essential for its antidepressant effects, and that the [2R,6R]-HNK enantiomer exerts behavioural, electroencephalographic, electrophysiological and cellular antidepressant-related actions in mice. These antidepressant actions are independent of NMDAR inhibition but involve early and sustained activation of AMPARs [α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors]. We also establish that [2R,6R]-HNK lacks ketamine-related side effects. Our data implicate a novel mechanism underlying the antidepressant properties of [R,S]-ketamine and have relevance for the development of next-generation, rapid-acting antidepressants.
Major depressive disorder affects around 16 per cent of the world population at some point in their lives. Despite the availability of numerous monoaminergic-based antidepressants, most patients require several weeks, if not months, to respond to these treatments, and many patients never attain sustained remission of their symptoms. The non-competitive, glutamatergic NMDAR [N-methyl-d-aspartate receptor] antagonist [R,S]-ketamine exerts rapid and sustained antidepressant effects after a single dose in patients with depression, but its use is associated with undesirable side effects. Here we show that the metabolism of [R,S]-ketamine to [2S,6S;2R,6R]-hydroxynorketamine [HNK] is essential for its antidepressant effects, and that the [2R,6R]-HNK enantiomer exerts behavioural, electroencephalographic, electrophysiological and cellular antidepressant-related actions in mice. These antidepressant actions are independent of NMDAR inhibition but involve early and sustained activation of AMPARs [α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors]. We also establish that [2R,6R]-HNK lacks ketamine-related side effects. Our data implicate a novel mechanism underlying the antidepressant properties of [R,S]-ketamine and have relevance for the development of next-generation, rapid-acting antidepressants.

![General Douglas MacArthur [1944] General Douglas MacArthur [1944]](https://upload.wikimedia.org/wikipedia/commons/0/0e/Douglas_MacArthur_lands_Leyte1.jpg)