Posted on Friday 27 May 2016
When I began to look at Clinical Trial reports a few years back, I had to relearn how to approach our literature. The studies are now industry funded, and that turned out to mean that the pharmaceutical companies control every step of the process, resulting in distorted and unreliable efficacy and side effect profiles. One might think that with all of the focused attention and attempts at reform, things might’ve changed more. But the beat just goes on.
Thase ME, Mahableshwarkar AR, Dragheim, M, Loft H, and Vieta E.European Neuropsychopharmacology. Mar 25, 2016 [Epub ahead of print]2014/2015 Impact Factor 4.369
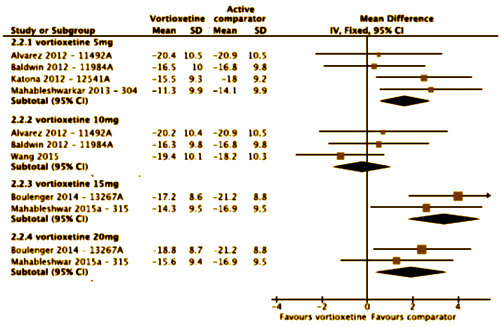
The efficacy and safety of vortioxetine, an antidepressant approved for the treatment of adults with major depressive disorder (MDD), was studied in 11 randomized, double-blind, placebo-controlled trials of 6/8 weeks? treatment duration. An aggregated study-level meta-analysis was conducted to estimate the magnitude and dose-relationship of the clinical effect of approved doses of vortioxetine [5-20mg/day]. The primary outcome measure was change from baseline to endpoint in Montgomery-Åsberg Depression Rating Scale [MADRS] total score. Differences from placebo were analyzed using mixed model for repeated measurements [MMRM] analysis, with a sensitivity analysis also conducted using last observation carried forward. Secondary outcomes included MADRS single-item scores, response rate [≥50% reduction in baseline MADRS], remission rate [MADRS ≤10], and Clinical Global Impressions scores. Across the 11 studies, 1824 patients were treated with placebo and 3304 with vortioxetine [5mg/day: n=1001; 10mg/day: n=1042; 15mg/day: n=449; 20mg/day: n=812]. The MMRM meta-analysis demonstrated that vortioxetine 5, 10, and 20mg/day were associated with significant reductions in MADRS total score [Δ-2.27, Δ-3.57, and Δ-4.57, respectively; p<0.01] versus placebo. The effects of 15mg/day [Δ-2.60; p=0.105] were not significantly different from placebo. Vortioxetine 10 and 20mg/day were associated with significant reductions in 9 of 10 MADRS single-item scores. Vortioxetine treatment was also associated with significantly higher rates of response and remission and with significant improvements in other depression-related scores versus placebo. This meta-analysis of vortioxetine [5-20mg/day] in adults with MDD supports the efficacy demonstrated in the individual studies, with treatment effect increasing with dose.
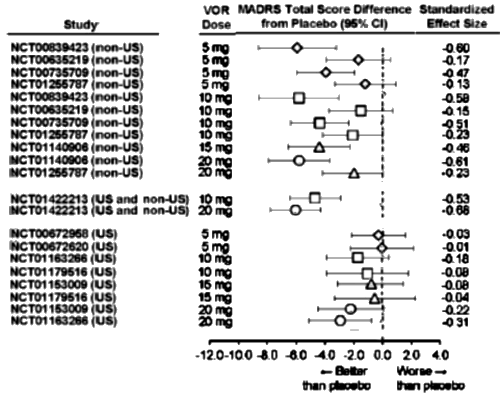
In the MADRS Total Score Differences [above], the forst plot abscissa is plotted as the raw difference. While that’s a legitimate way to show Effect Size, the units are unfamiliar, at least to me. In the far right column, they show the more familiar Standardized Effect Size [Mean Difference ÷ Standard Deviation] roughly scaled as 0.25=weak, 0.50=moderate, and 0.75=strong. Only one of the US sites reaches something that might remotely be clinically significant.
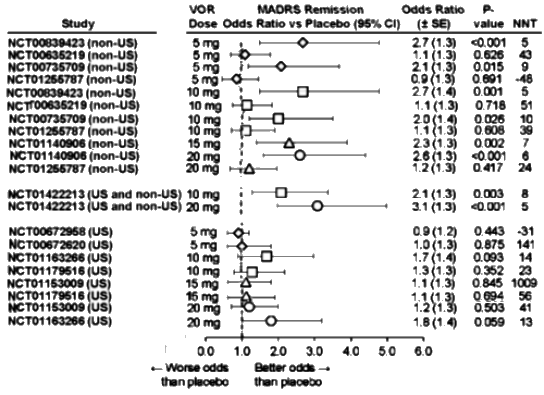
The MADRS Remission data is similar: Dose response curve? Not so much. And in the US sites, nothing achieves significance [p<0.05] or has an NNT or an Odds Ratio that is the range of a clinically solid antidepressant.
This meta-analysis centers on the comparison between vortioxetine and placebo in the 11 individual studies and does not evaluate differences between vortioxetine and the active references [duloxetine and venlafaxine XR]. The results of the active references can be found in the publications for the individual studies. In two of the previous meta-analyses of the vortioxetine data, direct comparisons between vortioxetine and the active reference were included. Direct comparison of vortioxetine and the active reference is not appropriate, as the individual studies were not designed or powered to enable this comparison. Rather, the rationale for including an active reference in these studies was for the internal validation of the study design [i.e., assay sensitivity]. To evaluate the efficacy of vortioxetine relative to another antidepressant would require a study that is specifically designed for that purpose, that is, an active-comparator study. In addition, in the six studies that include an active reference, patients were excluded – for ethical reasons – if they had known hypersensitivity or a history of lack of response to previous treatment with the active reference, which introduces the potential for bias in favor of the active reference.
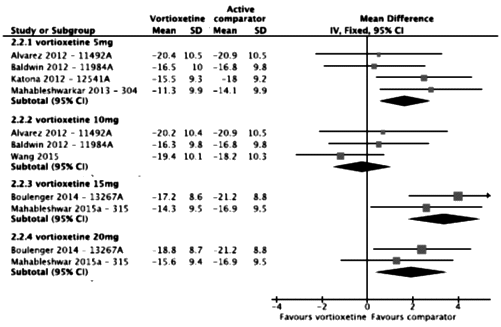
It’s sort of unusual to see an industry produced meta-analysis of their own clinical trials. I’ve given just a few examples where the way things are displayed or omitted falsely inflate the overall picture of how the drug performs. There are others, but even without the window dressing, this is a weak antidepressant on the best of days [if that]. I would speculate that this industry created meta-analysis is intended to neutralize the less than enthusiastic findings of the independent meta-analyses.



 David Healy
David Healy unexplored corners that occupied my Sunday.
unexplored corners that occupied my Sunday. 
 I first heard the phrase, "Sunlight is the best disinfectant" in Ben Goldacre’s initial Ted Talk [see
I first heard the phrase, "Sunlight is the best disinfectant" in Ben Goldacre’s initial Ted Talk [see