A commenter mentioned some studies, Harrow and Wunderink, and I was snappy. I have acquired snappiness writing this blog from instances where some piece of what I said is questioned and I answer only to find myself being asked to defend forced drugging, commitment laws, ECT, biomedicine, corporate greed, and any number of things that I really have nothing of worth to say anything about. I’ve identified when I feel it [my acquired snappiness]. It’s when I’m being cast in a role as a straw man. One would think I’d be used to it since transference is a central piece of the kind of therapy I did for a career. I think the difference is that being a straw man is just being a target [a narcissistic insensitive biomedical psychiatrist living in a mansion paid for with ill-gotten PHARMA profits]. Being a transference object as a therapist is in the service of learning something useful that might help understand and alter the course of a life. But I snapped at this commenter, perhaps prematurely, because my post was actually opposing the maintenance drug meme. Thinking about it later, as often as I’ve heard "Harrow and Wunderink" held up as a banner, I haven’t read either. So here’s one of them – Wunderink. The oft quoted paper is from 2013, but it’s a follow-up to an earlier study published in 2007:
Quantitatively, my experience with Schizophrenia was in a charity hospital in the mid-70s [the end of deinstitutionalization and the disappearance of Mental Hospitals]. Schizophrenia + Socio·Cultural deprivation is a hard way to live and that’s most of what I saw. Later, knowing my interest in the illness, I was referred some Schizophrenic patients along the way by former students. I had the idea that I could slowly aim for medication free life for them, but my experience wasn’t what I planned. I couldn’t bring it off. It was, instead, exactly what Wunderink et al reported in 2007:
by Wunderink L, Nienhuis FJ, Sytema S, Slooff CJ, Knegtering R, Wiersma D.
Journal of Clinical Psychiatry. 2007 68[5]:654-661.
OBJECTIVE: To compare the consequences of a guided discontinuation strategy and maintenance treatment in remitted first-episode psychosis in terms of relapse rates and functional outcome.
METHOD: The study was conducted in 7 mental health care organizations and the Department of Psychiatry of the University Medical Center Groningen in The Netherlands, covering a catchment area of 3.1 million inhabitants. A sample of 131 remitted first-episode patients, aged 18 to 45 years, with a DSM-IV diagnosis of schizophrenia or related psychotic disorder was included [i.e., all patients with a first psychotic episode from October 2001 through December 2002 who were willing to participate]. After 6 months of positive symptom remission, they were randomly and openly assigned to the discontinuation strategy or maintenance treatment. Maintenance treatment was carried out according to American Psychiatric Association guidelines, preferably using low-dose atypical antipsychotics. The discontinuation strategy was carried out by gradual symptom-guided tapering of dosage and discontinuation if feasible. Follow-up was 18 months. Main outcome measures were relapse rates and social and vocational functioning.
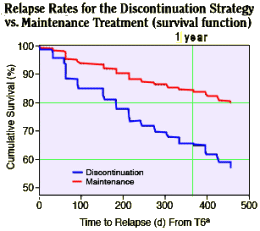 RESULTS:
RESULTS: Twice as many relapses occurred with the discontinuation strategy [43% vs. 21%, p = .011]. Of patients who received the strategy, approximately 20% were successfully discontinued. Recurrent symptoms caused another approximately 30% to restart antipsychotic treatment, while in the remaining patients discontinuation was not feasible at all. There were no advantages of the discontinuation strategy regarding functional outcome.
CONCLUSIONS: Only a limited number of patients can be successfully discontinued. High relapse rates do not allow a discontinuation strategy to be universal practice. However, if relapse risk can be carefully managed by close monitoring, in some remitted first-episode patients a guided discontinuation strategy may offer a feasible alternative to maintenance treatment. Further research is needed to find predictors of successful discontinuation.
It was uncanny reading this earlier article. I had looked it up because the later article was confusing about how these two groups differed [Maintenance vs Dose Reduction/Discontinuation] and I thought it might be clearer in this original study. It wasn’t. It was clinician’s choice. They concluded what I had been taught in training in the 1970s, and what I retired thinking in 2003 from experience. In my practice years, I was seeing less world-weary patients, and a relapse was a big deal, putting a strain on relationships, family, employment, and, of course, finances. So I had a few patients who ended up living medication-free, but it was way on down the road [and there was medication always on hand if needed]. That wasn’t how I wanted it to be. It’s just how it was. I ended my time in grade more in the aiming-for-medication-lite camp. And to be honest, the advantage over the old neuroleptics of the gentler atypicals kind of melts at lower doses – so I mostly ended up using the [generic] older drugs. Now on to Wunderink et al in 2013:
by Lex Wunderink, MD, PhD; Roeline M. Nieboer, MA; Durk Wiersma, PhD; Sjoerd Sytema, PhD; Fokko J. Nienhuis, MA
JAMA Psychiatry. 2013 70[9]:913-920.
IMPORTANCE: Short-term outcome studies of antipsychotic dose-reduction/discontinuation strategies in patients with remitted first-episode psychosis [FEP] showed higher relapse rates but no other disadvantages compared with maintenance treatment; however, long-term effects on recovery have not been studied before.
OBJECTIVE: To compare rates of recovery in patients with remitted FEP after 7 years of follow-up of a dose reduction/discontinuation [DR] vs maintenance treatment [MT] trial.
DESIGN: Seven-year follow-up of a 2-year open randomized clinical trial comparing MT and DR.
SETTING: One hundred twenty-eight patients participating in the original trial were recruited from 257 patients with FEP referred from October 2001 to December 2002 to 7 mental health care services in a 3.2 million–population catchment area. Of these, 111 patients refused to participate and 18 patients did not experience remission.
PARTICIPANTS: After 7 years, 103 patients [80.5%] of 128 patients who were included in the original trial were located and consented to follow-up assessment.
INTERVENTION: After 6 months of remission, patients were randomly assigned to DR strategy or MT for 18 months. After the trial, treatment was at the discretion of the clinician.
MAIN OUTCOMES AND MEASURES: Primary outcomewas rate of recovery, defined as meeting the criteria of symptomatic and functional remission. Determinants of recovery were examined using logistic regression analysis; the treatment strategy [MT or DR] was controlled for baseline parameters.
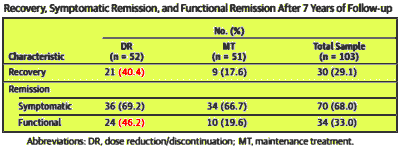
RESULTS: The DR patients experienced twice the recovery rate of the MT patients [40.4%vs 17.6%]. Logistic regression showed an odds ratio of 3.49 [P = .01]. Better DR recovery rates were related to higher functional remission rates in the DR group but were not related to symptomatic remission rates.
CONCLUSIONS AND RELEVANCE: Dose reduction/discontinuation of antipsychotics during the early stages of remitted FEP shows superior long-term recovery rates compared with the rates achieved with MT. To our knowledge, this is the first study showing long-term gains of an early-course DR strategy in patients with remitted FEP. Additional studies are necessary before these results are incorporated into general practice.
I’ve shown their data looking at the traditional endpoint – relapse rate. It’s interesting. When they looked at the five years that followed their original two year study, a couple of years after that first study, the groups equalized. I’m not sure we can make a lot out of the difference in that last year where there’s a possible reversal so I’ll leave that to you to ponder.
No relapse occurred in 36 patients [34.9%], 20 of whom were in the DR group [38.5% of all DR patients] and 16 in the MT group [31.4% of all MT patients]. The number of patients with a certain number of relapses in the DR [range, 0-5] and MT [range, 0-8] groups did not differ significantly [Pearson χ2 6 = 4.96; P = 0.55].
But what happened in the two years after their initial study looks rock solid to me. However, the thing that distinguishes this study is that they had looked not only at relapse rate, but at parameters correlated with functional recovery. My added background colors show the phases in this follow-up study: violet, the original two year study; any-icky-green, the follow-up to seven years total; dark-icky-green, the period where they looked at their symptomatic and functional parameters. And here’s the part of this study that people are impressed with [in red]:
And it is impressive. They more than doubled the Recovery and Functional Remission Categories [Categories are not exclusive which is why the sums don’t add up]. So how did they measure these characteristics?
Symptoms were assessed with the Positive and Negative Syndrome Scale [PANSS]. The PANSS was used to measure observer-rated severity of symptoms during the preceding week, as well as during the past 6 months.
Social functioning was assessed with the Groningen Social Disability Schedule [GSDS], a semistructured investigator based interview measuring disabilities in social functioning in 8 domains [7 of which were included in this study] over the past 4 weeks, as well as during the past 6 months. The 7 domains are self-care, housekeeping, family relationships, partner relationships, relationships with peers, community integration, and vocational functioning. The parenthood domain was omitted because of limited applicability. A disability is rated by the investigator on a 4-point scale: none [0], minimal [1], obvious [2], and serious [3].
Definitions of Recovery, Symptomatic Remission. Relapse, and Functional Remission
Criteria for recovery were met when patients had symptomatic and functional remission for at least 6 months at the 7-year follow-up. Criteria for symptomatic remission were adopted from Andreasen et al. All relevant PANSS item scores have to be 3 [mild] or less on a scale ranging from 1 [not present] to 7 [severe] during an observational period of 6 months. Patients were assessed retrospectively for any symptomatic re- lapse occurring during this period. A symptomatic relapse was defined as an exacerbation of symptoms during at least 1 week with at least 1 relevant PANSS item score above 3 [mild]. Any relapse in symptoms during the 6 months preceding the assessment prevented the individual from being categorized as recovered at the time of the assessment.
According to generally accepted views, functional remission implies proper social functioning in the main domains of everyday life. The 7 domains of the GSDS included in the present study adequately represent these domains. A patient with functional remission should function adequately in all 7 domains with none or only a minimal disability in any of them [not allowing a score of 2 or 3 on any GSDS domain]. Patients were considered to have functional remission if, during an observational period of 6 months before assessment, all functional domain scores remained at 1 or lower.
I named this post "persistence" because [1] Wunderink et al persisted in following their cases and [2] you’re going to need to persist because I’m not done yet.
This is the part that’s hard to follow: What was the difference in medication use/dose between the DR [Dose Reduction/Discontinuation] and MT [Maintenance Treatment] groups? Complicated numbers, complicated analyses, complicated graphs. It’s available for all to read in full. Here’s the take home: During the two years at the end, in the DR Group, 11/52 [21%] were off all medications, and in the MT group, 6/51 [12%] were off all medications. Of those remaining on medications, the [Haldol Equivalent] doses were DR = 2.79 [2.21]mg and MT = 4.08 [4.03]mg with P=0.07 [there’s much more in the paper about this, but it’s not blogger-friendly].
There were some other things of interest. They looked at factors that might predict a good outcome in each of the categories [Recovery, Symptomatic Remission, and Functional Remission]. This is their table trimmed to only show factors with at least one significant correlation [in red; circled values were significant by step-wise logistical regression]:
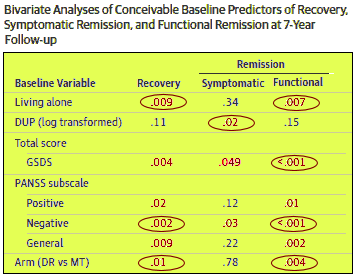
Mostly, the usual suspects, but was interesting that the solid predictor of Symptomatic Remission was DUP [Days of Untreated Psychosis] – not Arm [DR vs MT]. I’ve had that thought myself – that if a person stays psychotic for a long time without initial treatment, they’re much less likely to respond to medication [as in Dan Markingson who was psychotic for months before his mother could get him home]. My guess is that with prolonged psychosis, paranoid beliefs set in and grow. Elaborate paranoid beliefs are the least treatable of the Schizophrenic symptoms.
I’m not going to paraphrase the author’s conclusions to this study. I think they do a fine job all by themselves, and I’m not interested in getting in their way. Hopefully this summary of their findings will stimulate your curiosity to read their thoughtful remarks. Remember, this paper and topic aren’t about ideology, mental health specialty, or anti-this-or-that. They’re about the best case treatment of First Episode Schizophrenia/Psychosis. For myself, I enjoyed this paper. I think I had felt a little guilty that my own bias in seeking the lowest possible antipsychotic dose [or none] had lead to some un-necessary psychotic breaks. One can always use a little reassurance along the way. I was also comforted that though my own cases were few, their experience was very similar to my own in carrying through with the Dose Reduction/Discontinuation mindset. It may be a goal, but in patient management, the clinical reality is always the compass that leads the way. And you don’t always get what you want…
They ended with:
Of course, only one study indicating advantages of a DR strategy in patients with remitted FEP is not enough evidence in such an important matter. However, these results merit replication by other research groups.
Which is absolutely correct. I hope that’s happening somewhere. My own take on this is a more general and personal point. When I was in Internal Medicine, as much as I enjoyed the science of things, there was part of the practice that I didn’t like at all. The majority of the people that showed up with symptoms did not have a physical illness explaining why they came or were sent. And while most of them just needed some reassurance, there were a surprising number who obviously had something significantly wrong. And there would never be a future time when I could do anything about that as a busy Internist Specialist [which is what I was]. In those days, I was in a lull period – a military doctor on a [healthy] overseas Air Force base – and I did have plenty of time. So I started talking to those patients who had "negative" work-ups, and was amazed by what lay behind their symptoms. I didn’t know enough to always know what to do, but I could do a lot even in my psyche-naive state. I guess I got hooked. All those years ago, I decided that the essence of good medical care was providing someone to "follow along" and "think along" with any chronic illness, whether it be medical or psychiatric. And I still believe that forty plus years later.
So I gravitated to psychiatry. And just after I had finished my training, Psychiatry and Managed Care decided I shouldn’t take the time to do that anymore. Now they even want me to do Collaborative Care and not even see the patients. I said and say no thanks to all of that – and I’m glad I made that decision. In regard to the topic of this paper – First Episode Schizophrenia is a mega-big-deal in the life of a person and a family. These patients don’t just need Guidelines, they need a person to go along on the ride. It helps a lot if that person is not otherwise connected with their life and knows a whole lot about their illness. I had no interest in having some watchdog telling me what to do based on an accounting program, or some KOL premetabolizing medical information. So I never dealt directly with Insurance companies, and I adjusted my fees to fit the patient if I could. I dropped out of the APA from lack of interest [and relevance].
I think there’s a lot more to following a patient with Schizophrenia than just the medication prescription, more than something like CBT, or psychoanalysis, or anything that can be put down as a procedure. Whatever this condition is, it’s a hard way to be alive and the patients deserve whatever consistent ongoing benevolent interpersonal help they can rely on [and tolerate]. And it sometimes needs to be without an ending.
[3] So there’s my third reason to call this post "persistence"…
 Well, I reread the editorial and series this morning, and if anything, they were worse – bordering on polemics. I tried to cut them a little slack because they’re internists and not psychiatrists. Psychiatry has been on the leading edge of the kind of problems COI can lead to for decades. Maybe, I thought, they’re just naive and not so in touch with the kind of fire they’re playing with here. They’re civilians, whereas we psychiatrists are veterans – knowing first hand how this COI thing can grow into a metastacizing cancer. But then I was alerted to this story about the Vioxx debacle by Bioethicist Arthur Schafer [University of Manitoba]. Editor Jeffrey Drazen should be a veteran after Vioxx [see also in Wikipedia]:
Well, I reread the editorial and series this morning, and if anything, they were worse – bordering on polemics. I tried to cut them a little slack because they’re internists and not psychiatrists. Psychiatry has been on the leading edge of the kind of problems COI can lead to for decades. Maybe, I thought, they’re just naive and not so in touch with the kind of fire they’re playing with here. They’re civilians, whereas we psychiatrists are veterans – knowing first hand how this COI thing can grow into a metastacizing cancer. But then I was alerted to this story about the Vioxx debacle by Bioethicist Arthur Schafer [University of Manitoba]. Editor Jeffrey Drazen should be a veteran after Vioxx [see also in Wikipedia]: In sum, almost no one emerges with much credit from the saga of the Cox-2 inhibitors. The drug companies which massively marketed the drugs, both to doctors and directly to consumers, made billions of dollars; but, when the facts eventually emerged, the companies experienced a serious loss of public trust. Merck, in particular, is now facing a staggering number of expensive law suits. The company continues to insist that it took all reasonable measures to determine whether Vioxx carried undue cardiovascular risks and is defending its conduct in all of these law suits. Medical journals and their editors, in particular the NEJM and its editor Dr. Jeffrey Drazen, were seen by some critics as being incompetent at best and collusive at worst in what turned out to be a terrible human tragedy…


![Plato's Academy [Pompeii Mosaic] Plato's Academy [Pompeii Mosaic]](http://1boringoldman.com/images/academy.gif) And so it goes as my bias generates contrarian counters to his arguments. Back in the days of the Academy in Ancient Greece, a time when they fell in love with the rules of argument and logic, they initially thought they could reach absolute truth with their debates. So they studied logic and cataloged wrong arguments as the fallacies. Then along came questions of bias, and motive, and later even unconscious motive. And the sacred olive groves of Plato’s Academe [and later Aristotle’s Lyceum] moved from the quest for dogmatic truth to becoming cloistered havens for skepticism and a questioning attitude. And Academia has been under siege ever since [with arguments not unlike the ones coming from this NEJM series].
And so it goes as my bias generates contrarian counters to his arguments. Back in the days of the Academy in Ancient Greece, a time when they fell in love with the rules of argument and logic, they initially thought they could reach absolute truth with their debates. So they studied logic and cataloged wrong arguments as the fallacies. Then along came questions of bias, and motive, and later even unconscious motive. And the sacred olive groves of Plato’s Academe [and later Aristotle’s Lyceum] moved from the quest for dogmatic truth to becoming cloistered havens for skepticism and a questioning attitude. And Academia has been under siege ever since [with arguments not unlike the ones coming from this NEJM series]. In a recent discussion about the origin of the chemical imbalance notion of depression [see
In a recent discussion about the origin of the chemical imbalance notion of depression [see  RESULTS: Twice as many relapses occurred with the discontinuation strategy [43% vs. 21%, p = .011]. Of patients who received the strategy, approximately 20% were successfully discontinued. Recurrent symptoms caused another approximately 30% to restart antipsychotic treatment, while in the remaining patients discontinuation was not feasible at all. There were no advantages of the discontinuation strategy regarding functional outcome.
RESULTS: Twice as many relapses occurred with the discontinuation strategy [43% vs. 21%, p = .011]. Of patients who received the strategy, approximately 20% were successfully discontinued. Recurrent symptoms caused another approximately 30% to restart antipsychotic treatment, while in the remaining patients discontinuation was not feasible at all. There were no advantages of the discontinuation strategy regarding functional outcome.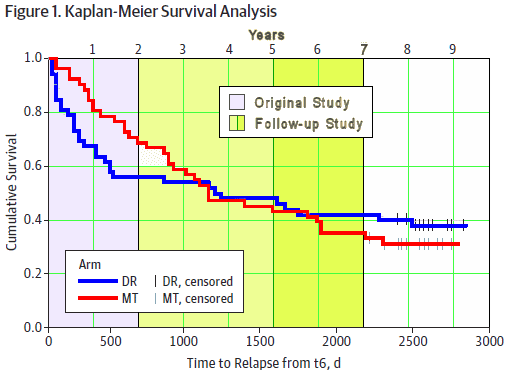

 There were Prolixin Clinics, and visiting nurses who took the medication to the patients. It seemed to me that it sounded like a better idea than it was, at least in the inner-city environment where I worked. Besides the Big Brother [1984] feel to the scheme, many of the patients were as unlikely to show up for their monthly injection as they were to take their oral medication. In recent history, the Long Acting Injectable [LAI] Atypical Antipsychotics tend to be released late as the drugs go off-patent, presumably as a patent extension ploy [above].
There were Prolixin Clinics, and visiting nurses who took the medication to the patients. It seemed to me that it sounded like a better idea than it was, at least in the inner-city environment where I worked. Besides the Big Brother [1984] feel to the scheme, many of the patients were as unlikely to show up for their monthly injection as they were to take their oral medication. In recent history, the Long Acting Injectable [LAI] Atypical Antipsychotics tend to be released late as the drugs go off-patent, presumably as a patent extension ploy [above].  The drug here is Abilify Maintena®, the depot form of Abilify® [Aripiprazole]. So the study brings up two semi-separate issues: the use of depot medications to deal with medication compliance and the treatment of a first episode of Schizophrenia with a fixed dose of depot medication. This is a stopping place, but there’s more to come…
The drug here is Abilify Maintena®, the depot form of Abilify® [Aripiprazole]. So the study brings up two semi-separate issues: the use of depot medications to deal with medication compliance and the treatment of a first episode of Schizophrenia with a fixed dose of depot medication. This is a stopping place, but there’s more to come…