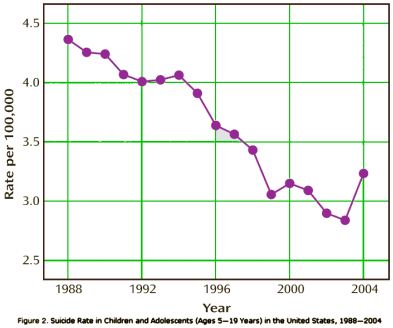
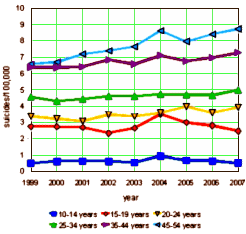
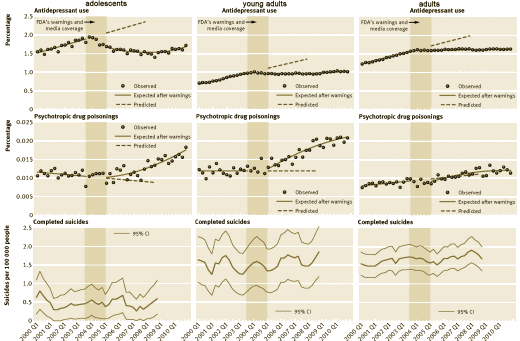
The 1991 FDA hearing about SSRIs and suicidality was principally focused on Prozac and pitted case reports against the Clinical Trial data. My recollection is that the general thought was that this was seen as a campaign initiated by the Scientologists and it didn’t have a major impact at the time. But the second time around over a decade later was another matter. It was at what we might now call the apogee of the Age of Pharmacology [in that day still a rising star]. This time, the focus was on children and adolescents; there was a damning FDA meta-analysis; and many more cases. The Black Box Warning was born:

The war on the Black Box Warning began immediately, and has been unrelenting for the decade that followed – saying that the analyses showing increased suicidality were wrong or that the Black Box Warning and the publicity caused doctors and patients to withhold needed treatment leading suicide attempts to actually increase. If there is a single consistent point from all this flap, it was a consensus that the rate of prescribing antidepressants to children and adolescents has fallen as a result after the warning.
The many attempts to discredit or undo the Black Box Warnings have involved multiple tries at reanalysis of the Clinical Trials and some attempts to show an adverse effect using large databases from a variety of sources. This most recently published study from the Harvard Pilgrim Health Care Institute [
Changes in antidepressant use by young people and suicidal behavior after FDA warnings and media coverage: quasi-experimental study] attempted to query the data from a number of commercial regional Health Care plans. There are a number of confounding factors in taking this approach, some having to do with the data itself. The most explicit coding in the ICD-9 for suicide attempts are in the E-codes [external events] –
deliberate self harm. However in many of the Healthcare plans, E-coding is very spotty ["
However, insurance claims databases such as Medicare have low rates of E-code completeness, presumably because the billing software used by many hospitals removes E-codes since they have no relevance for hospital payments"]. In 2010, Patrick et al studied several datasets trying to find a way around this problem:
by Amanda R. Patrick, Matthew Miller, Catherine W. Barber, Philip S. Wang, Claire F. Canning and Sebastian Schneeweiss
Pharmacoepidemiology and Drug Safety. 2010 19:1263–1275.
Context: Suicidal behavior has gained attention as an adverse outcome of prescription drug use. Hospitalizations for intentional self-harm, including suicide, can be identified in administrative claims databases using external cause of injury codes [E-codes]. However, rates of E-code completeness in US government and commercial claims databases are low due to issues with hospital billing software.
Objective: To develop an algorithm to identify intentional self-harm hospitalizations using recorded injury and psychiatric diagnosis codes in the absence of E-code reporting.
Methods: We sampled hospitalizations with an injury diagnosis [ICD-9 800–995] from two databases with high rates of E-coding completeness: 1999–2001 British Columbia, Canada data and the 2004 US Nationwide Inpatient Sample. Our gold standard for intentional self-harm was a diagnosis of E950-E958. We constructed algorithms to identify these hospitalizations using information on type of injury and presence of specific psychiatric diagnoses.
Results: The algorithm that identified intentional self-harm hospitalizations with high sensitivity and specificity was a diagnosis of poisoning, toxic effects, open wound to elbow, wrist, or forearm, or asphyxiation; plus a diagnosis of depression, mania, personality disorder, psychotic disorder, or adjustment reaction. This had a sensitivity of 63%, specificity of 99% and positive predictive value [PPV] of 86% in the Canadian database. Values in the US data were 74, 98, and 73%. PPV was highest [80%] in patients under 25 and lowest those over 65 [44%].
Conclusions: The proposed algorithm may be useful for researchers attempting to study intentional self-harm in claims databases with incomplete E-code reporting, especially among younger populations.
Using these two databases with high levels of E-code completion, they derived an algorithm that they felt was an adequate "proxy" that correlated well with the explicit E-codes for deliberate self harm: "a diagnosis of poisoning, toxic effects, open wound to elbow, wrist, or forearm, or asphyxiation; plus a diagnosis of depression, mania, personality disorder, psychotic disorder, or adjustment reaction."
In February 2014, Lu et al [authors of the recent article in the BMJ] wrote a letter to the editor about another
article from Vanderbilt that had used E-codes and psychiatric diagnosis as an indicator of suicide attempts. They pointed out the unreliability of WE-code reporting in commercial databases and illustrated the point with some examples of their own:
Letter to the Editor
by Christine Y. Lu, Christine Stewart, Ameena T. Ahmed, Brian K. Ahmedani, Karen Coleman, Laurel A. Copeland. Enid M. Hunkeler, Matthew D. Lakoma, Jeanne M. Madden, Robert B. Penfold, Donna Rusinak, Fang Zhang, and Stephen B. Soumerai
Pharmacoepidemiology and Drug Safety. 2014 23[2]:218-220.
We advise caution in applying the claim-based algorithm developed by Callahan et alet al. method uses external cause of injury codes [E-codes] in combination with diagnosis codes for poisoning derived from the International Classification of Diseases, ninth revision, Clinical Modification [ICD-9-CM] coding scheme to identify hospitalizations for suicide attempts. In recent years, there has been considerable concern that suicidal behavior is a potential adverse outcome of prescription drug use such as antidepressant and anticonvulsant agents. Nonfatal, deliberate self-harms resulting in emergency department treatments and hospitalizations can be identified in administrative databases using E-codes. These codes are part of the ICD-9-CM and are used to provide information about the cause and intent of an injury or poisoning. E-coding is mandatory in about half of US states, and the completeness of E-codes in state hospital discharge databases typically exceeds 90%. As part of a study of effects of safety warnings on antidepressant use and suicidality in youth, we assessed the completeness of E-codes in commercial health plan databases.
METHODS: Our analysis included 10 geographically distinct healthcare organizations in the Mental Health Research Network [MHRN] within the Health Maintenance Organization Research Network [HMORN]. The health plans had a combined population of nine million enrollees in 2010. This analysis was part of a longitudinal study of effects of Food and Drug Administration warnings for antidepressants and suicidality in youth that was approved by the institutional review board of each participating organization…
We calculated the completeness of E-codes, defined as the proportion of encounters with an injury/poisoning ICD-9-CM code that had a valid E-code indicating the cause for the encounter. As in prior research, we identified hospitalizations and emergency department visits with a primary or secondary diagnosis of injury/poisoning. We focused on injuries that are likely methods of deliberate self-harm: open wound injuries, superficial injuries, and poisonings. Because E-code collection and reporting requirements vary temporally and by region, we assessed E-code completeness rates from 2000 to 2010 by MHRN site and care setting. A V-code [supplemental information about factors influencing health service use] was introduced in 2005 indicating suicidal ideation [ICD-9: V62.84]. In a sensitivity analysis, we calculated E-code completeness, while also including V62.84 that could be used in place of E-codes to identify a suicidal related encounter.
RESULTS: Figure 1 presents E-code completeness rates in emergency department and hospital settings over time. E-code completeness varied widely across study sites [e.g., ranging from 7% to 92% in the emergency department setting in 2010], across treatment settings [e.g., ranging from 7% to 56% at one study site in 2010], and across years [e.g., ranging from 36% in 2000 to 92% in 2010 at one study site]. Only two sites had consistent, reasonable levels of E-code recording over this period [ranging from 65% to 82%]. Our investigation indicates that the suicidal ideation code did not substitute or compensate for lack of E-codes with injury/poisoning diagnoses.
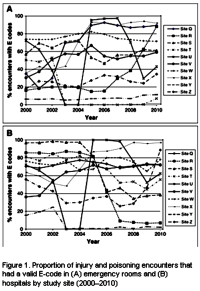
Proportion of injury and poisoning encounters that had a valid E-code in [A] emergency rooms and [B] hospitals by study site [2000–2010]
COMMENT: In our analysis of VDW data from 10 MHRN sites between 2000 and 2010, we found that E-code completeness varied across study sites, across treatment settings, and across years of observation. There are several possible reasons for the low rates of and/or variability in E-code completeness observed: E-codes have no relevance for payments; not all diagnosis codes are transformed into the VDW from source data; and recording practice for E-codes may vary across sites, possibly because of state regulations, health plan policies, or the clinical software used. The incompleteness we observed in this study limits the usefulness of the available E-coded data. Other studies also found high missingness of E-codes in hospital and emergency department settings.
Despite the high positive predictive value of 85% reported by Callahan et al. there are two issues relating to the use of the algorithm: [i] the completeness of E-codes in the dataset and [ii] the dependence on valid E-coded data.
We agree with Callahan et al. that it is important to develop and use alternative diagnosis codes that can identify suicide attempts. In the absence of complete E-codes, Patrick et al. developed and tested algorithms for identifying hospitalizations for deliberate self-harm in a population aged 10 years and over. This study used the US National Inpatient Sample data and data from British Columbia; both data sources had E-code completeness rates above 85 %. The gold standard for deliberate self-harm was defined as hospitalizations with a diagnosis of E950-958. Patrick et al. found that an algorithm combining diagnoses for psychiatric disorders [including depression] and injury/poisoning can produce a positive predictive value as high as 87.8% for identifying hospitalizations for deliberate self-harm [with specificity of 99.4% and sensitivity of 57.3%].
If you’ve been scanning along here sleepily, it’s time to sit up and take notice. After illustrating the E-code problem, they question Patrick et al’s solution [the algorithm above]…
In the context of our longitudinal study on the impact of Food and Drug Administration warnings on antidepressant use and subsequent suicidality in youth, using Patrick’s algorithm may introduce ascertainment bias because rates of depression diagnosis declined subsequent substantially after the warnings.
If you’re following this, they’re about to jettison Patrick’s algorithm because the rate of diagnosing pediatric depression was reported as being decreased [mostly in primary care]. Here are the references they cite for this comment:
-
-
I call foul! These are Eli Lilly funded articles by Eli Lilly funded authors from a period when there was an all out campaign against the warning. They proselytize calling for policy changes based on… I’ll stop rather than rant. The articles are there to read and are typical for the kind of PHARMA invasion of scientific literature that we’re all raving about. Take a look. The idea that they represent ascertainment bias is ludicrous. They represent something else – Bad Science by Bad Pharma. A rational interpretation is that the Black Box Warning put some badly needed brakes on a runaway overmedication epidemic. [see
pretty loud coi…,
tortured numbers…, etc]
. Moving along…
A diagnosis of “poisoning by psychotropic agents” alone outperformed other injury/poisoning types; its positive predictive value was 79.7% with specificity of 99.3% and sensitivity of 38.3%.
So here they go back to Patrick’s article that compiled a bunch of algorithms to test and pick one with a much weaker sensitivity and predictive value and many other problems.
While psychotropic drug poisoning underestimates rates of suicide attempts because of its low sensitivity, it can be useful for detecting suicide attempts in study settings that have low or inconsistent E-code rates over time. Such consistency is required for longitudinal analyses of trends in deliberate self-harms.
In summary, our analysis confirmed that E-codes were substantially incomplete in commercial insurance claims databases. We observed that E-code completeness varied widely across MHRN sites, across treatment settings, and over time. Completeness improved at some sites and deteriorated at other sites. Psychotropic drug poisonings may be useful for identifying deliberate self-harm requiring hospitalization in commercial plan databases when E-codes are missing…
So now on to back to the article for another look…