I first heard that PHARMA was shutting down its CNS drug development when I read Stephen Stahl let loose with a rant heard far and wide in August 2011 [see
myopia – uncorrected…]. So I thought it was ironic that a few days later, I read in the
American Journal of Psychiatry about a new Atypical Antipsychotic being introduced –
Latuda® [see
ought to know by now…]. On the by-line, that paper had seven
Sunovion employees [manufacturer of
Latuda®], one Quintiles employee [a Clinical Research Organization], and two KOLs on the Sunovion payroll [one of whom was a professional medical writer who may have written the paper]. Digging further into the FDA Approval documents, things looked odd from any angle [from
or both…]:
When I started nosing around, I found all kinds of interesting and strange things. There was wide variability in efficacy among countries and doses.
No matter where I looked, there was something amiss, including Lurasidone bringing up the rear in a comparative meta-analysis of antipsychotic efficacy [
the mother of meta…]. One thing I recall thinking back then was that the most functional part of
Sunovion was their marketing department. Looking at clinicaltrials.gov, they had already started all of the trials needed to aim for new indications [eg Bipolar Disorder] before even being initially approved for Schizophrenia [see
echo echo echo echo echo echo echo… and
creepy…]. They’d learned the formula for success from their predecessors.

I didn’t give
Latuda® much thought after that. I had noticed in their signature
before-and-after ads that
Latuda® apparently moves the part from one side of the head to the other
like a mirror. So when one of those ads popped up, I always noticed. But otherwise,
Latuda® was off my radar. And then it was February 2014, and I looked at the
American Journal of Psychiatry and there were two articles about
Latuda® Clinical Trials in the same issue
and there was an
editorial about it. You guessed it, a Bipolar Disorder indication. Then, I wrote [from
or both…]:
Both articles were glowing. I remembered how informative the
Drugs@FDA site had been in looking over the original
Latuda® approval in 2011, so I went looking for their new indication, but there was nothing there except the label change in January 2013 adding the new approval. No new Medical Review in sight. I’d had splendid success with Freedom of Information Act [FOIA] requests to the FDA. Within a couple of weeks, I’d gotten the package of documents for
Prozac®,
Paxil®, and
Zoloft® from earlier requests along the way. So I thought I’d just put in an FOIA request and find out the back story on the
Latuda® indication "creep." A couple of days later, I was writing this [
on time…]:
Tuesday, after a long morning working in the clinic, I was awakened from a recovery nap by a mid-afternoon phone call. It was the FDA calling. The lady on the phone told me she’d seen I’d made other requests, but they were for "older drugs" [I wasn’t liking how this was sounding so far]. She said it was different for recent approvals. I didn’t follow all of what she was saying, but she seemed to be trying to dissuade me from pursuing my request. She said it had to go through several processes one of which was "disclosures" and there might be a charge. Did I still want to stay in the queue? By this time I was awake enough to say, "Sure. How long is the usual wait?" She said "18 to 24 months. You know, we get over 3000 requests every year." long pause. I said "Okay" [having given up with taking out my frustrations with big systems on small bureaucrats long ago]. But when I was fully awake and thought about it, I think I learned something, and have to revise my theory. My now fading enthusiasm for the FDA had been based on experiences where what I was requesting was old hat, and just required copying a disk from somebody else’s previous request. The fantasy that I could get the information on a recent approval in a timely fashion was naive. This drug is going to be well down the line in it’s patent life before I ever see that report, just like before.
So why am I writing about Latuda® right now? It’s because a reminder I set a year ago on my calendar after that phone call popped up to let me know that my FOIA request went in a year ago this month [and so far, silence from the FDA]. I’m not grandiose enough to think that the FDA has people sitting around poised to respond to my every whim. So I’m not surprised at the year of silence. And my calendar is dutifully set for another reminder at this time next year [which will be the advertised "24 months"]. And I recognize that my earlier successes were on older drugs that have probably been requested by big law firms involved in all the legal wranglings about Prozac®, Paxil®, and Zoloft® and were already available. So I’ll wait patiently to see if the information about the indication sprawl for Latuda® ever reaches our North Georgia mountains for me to peruse.
There’s a point to this narrative. I went looking for how Latuda® was doing on the market. What about sales? That took me to various business sites, and somewhere on that road I read that Latuda® prescriptions were up 29% since the Bipolar approval. I finally ended up on the Dainippon Sumitomo Pharma Co., Ltd. site [Sunovion is owned by this Japanese firm]. And going through the yearly financial reports, I came up with this table [ballpark figures]:
YEAR
|
US SALES
|
| 2011 |
$60 M |
| 2012 |
$136 M |
| 2013 |
$385 M |
| 2014 |
$740 M |
It looks like it’s headed for "Blockbuster" status next year [$1 B/YR]. So here’s a late-comer that dribbled out of the pipeline just as the spigot was being turned off. It’s competing with its atypical predecessors and the first generation antipsychotics, all of which are available as less expensive generics. And it’s at the bottom of the efficacy list in the only meta-analysis that I know of that includes it [see the mother of meta…]
:
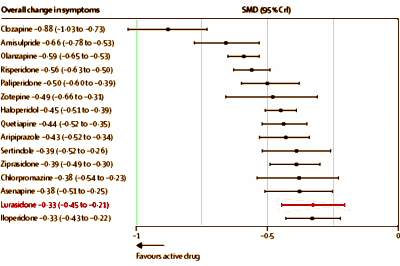
And yet it got through the FDA with an 11th hour intervention by the FDA Director. It had three published Clinical Trial articles and an editorial that made it into the prestigious American Journal of Psychiatry. So it seems to be having an unusually charmed life, and is selling like hotcakes. As I said, these Sunovion people really seem to know how to sell some drugs [Sales Force Report: A Walk on the Sales Side].
That point I said I was aiming for is that while Latuda® is zooming up the ladder in sales and usage, I’m measuring my ability to find out about its safety and efficacy literally in years. So long as that’s the case, it’s going to be the pharmaceutical industry’s sales force that will exert the major influence on which drugs are prescribed – rather than the plain clinical facts. There’s no reason in the world for the FDA data seen by the reviewers to need anything except a postage stamp to get it to anyone else qualified to take a look. If there’s commercially confidential information on a drug, don’t put it in the material submitted. If there’s data that needs to be anonymized, do it before the submission. The FDA has more important things to do than calling some old man in the middle of a perfectly good nap to try to talk him out of wanting what he wants. Leave the keeping secrets business for the people in Langley Virginia where it makes sense…
Afterthought: When I looked back over this, I realized I left out something that belongs here. I think new has a high value. There are plenty of patients who have cycled through many of the available meds and are shopping for something new. And Latuda® came after an empty space – was the last something new on the block punctuating the dry pipeline meme. And I would recommend reading that article on drug rep technology [Sales Force Report: A Walk on the Sales Side]…
 I didn’t give Latuda® much thought after that. I had noticed in their signature before-and-after ads that Latuda® apparently moves the part from one side of the head to the other like a mirror. So when one of those ads popped up, I always noticed. But otherwise, Latuda® was off my radar. And then it was February 2014, and I looked at the American Journal of Psychiatry and there were two articles about Latuda® Clinical Trials in the same issue and there was an editorial about it. You guessed it, a Bipolar Disorder indication. Then, I wrote [from or both…]:
I didn’t give Latuda® much thought after that. I had noticed in their signature before-and-after ads that Latuda® apparently moves the part from one side of the head to the other like a mirror. So when one of those ads popped up, I always noticed. But otherwise, Latuda® was off my radar. And then it was February 2014, and I looked at the American Journal of Psychiatry and there were two articles about Latuda® Clinical Trials in the same issue and there was an editorial about it. You guessed it, a Bipolar Disorder indication. Then, I wrote [from or both…]:
Thanks. I guess I live in a little bubble but I do not get that people (doctors) are not on to this yet. I appreciate your efforts.
http://www.madinamerica.com/2014/02/new-silver-bullet-lurasidone-story/
Sandra,
Thanks. We do live in a bubble. We think that because we pore over this stuff and see the big picture that the efforts of these blogs at the end of the galaxy.ought to make a difference. But if we did some math, our impact factor would go like this:
My theory is that if I say it enough times, I’m lowering that number – ergo “boring” old man…
see Sales Force Report: A Walk on the Sales Side for their impact factor theories…
It is frustrating and disappointing on many levels but there is this area of wealth in a system starved for resources.