Posted on Tuesday 17 May 2016
Under the Influence: The Interplay among Industry, Publishing, and Drug Regulation.
by Cosgrove, Vannoy, Mintzes, and Shaughnessy
As you can see from the opening quote, this article is about a lot more than just the specific papers or even the FDA Approval, it’s about the perversion of the scientific process in the industry funded RCTs, what the authors call ghost management [AKA total commercial control of the trial process from design and registration through the drafting of the paper for publication]. Being reminded of this paper, I recalled that when I first came across it I contacted the authors to get a copy and was told that they too had had a very hard time getting it published. It’s in a lower impact journal and it’s not available from the publisher on-line [an expensive proposition unless it’s part of grant or you’re involved with a well-heeled corporation]. So I recontacted them to find out if they had written about their publishing problems, and they hadn’t. But they mentionedf that they were disappointed with the paper’s reception, as was I. It’s a really strong and well documented article. Thinking back about our experience with the Paxil Study 329 RIAT article, the recent Citalopram deconstruction article, this Vortioxetine paper, the papers about the Tamiflu re-evaluation, and countless others, it’s apparent that there’s a common theme. So I want to revisit this Vortioxetine article later [I’ve found a way to get to it on-line], but first, I’d like to frame the common theme that unites these papers.
It’s a mighty hard row that my poor hands have hoed
My poor feet have traveled a hot dusty road
Out of your Dust Bowl and Westward we rolled
And your deserts were hot and your mountains were cold
I worked in your orchards of peaches and prunes
I slept on the ground in the light of the moon
On the edge of the city you’ll see us and then
We come with the dust and we go with the wind…
What unites these articles is obvious – the are contrarian. They say that somebody else’s work is wrong, and in these cases, wrong on purpose. They use that somebody-else’s data in reanalysis to reach a different, often opposite conclusion. They make a serious charge, so they deserve careful scrutiny sure enough. But in the case of all four articles, they’re not, primarily, opinion pieces. They’re data-based analyses that, by definition, render an opinion, but it’s an evidence-based opinion rather than speculative. Yet even after being subjected to a rigorous examination far exceeding that applied to the originals, they’re still hot potatoes. There’s obviously a fear of reprisal, legal challenges by the powerful sponsors of the originals. And there’s something else, the authors are under suspicion for having ulterior motives, conflicts of interest. The obvious commercial conflicts of interest in the originals are buried in small print, but the fact that the reanalysis articles are contrarian in and of itself seems grounds for such a charge.
-
data collection:
In each of these cases, just getting hold of the data was itself a daunting task, coming from reluctant sources in formats that made analysis difficult. -
funding:
This is largely unfunded research. If there is grant support, it’s often for the investigator without allowance for statistical or technical support, indirect costs, or publication expenses. -
heightened standards:
The burden of proof for contrarian articles is uniformly higher than for the originals. Likewise, requirements for freedom from conflicting interests are more stringent – more on the side of guilty until proven innocent. -
opinion rather than science:
Contrarian articles tend to be judged as opinions rather than scientific exploration – and biased opinion at that. -
accessibility:
Because they’re often in lower impact journals and have to rely on charity to be available online, they can get lost in an ivory tower or a dusty corner of the library either never catching the wind, or if they do, having a short hang time. -
seen as deprivation :
While their intent is health promoting, they can be seen as taking away something. -
no PR:
They’re not necessarily picked up by the news media, mentioned on the business pages, or reviewed in the professional trade journals
This is hardly a comprehensive list, just something off the cuff. But I think it captures the fact that these are hard articles to write and hard articles to publish. But that’s just the beginning. Often, getting them into the public and professional discourse is a another uphill climb. And while it’s tempting to see the difficulties as coming from evil opposing forces, and I’m certain that complaint is often justified, it’s also not the whole story. Contrarian literature in science is like that all on its own. We want to hear about breakthroughs, not breakdowns. That’s just the nature of things.


 There are times when being wrong is just fine. When I first read about Ben Goldacre‘s
There are times when being wrong is just fine. When I first read about Ben Goldacre‘s  COMPARE encountered in obtaining a protocol for one of the studies in their audit prompted us to implement it earlier…
COMPARE encountered in obtaining a protocol for one of the studies in their audit prompted us to implement it earlier…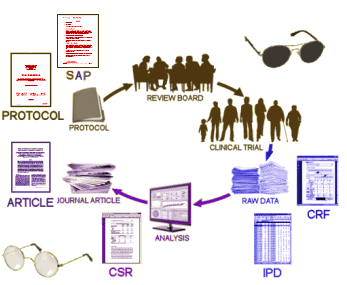
 In fact, if you took a statistics course, the teacher was likely a psychologist or a social scientist rather than someone from the math department. Statistical analyses are about conditional likelihoods [with the emphasis on conditional]. And De Groot is pointing out that, unlike the other mathematics, one absolute condition in confirmatory statistical analysis is blindness [with the emphasis on absolute].
In fact, if you took a statistics course, the teacher was likely a psychologist or a social scientist rather than someone from the math department. Statistical analyses are about conditional likelihoods [with the emphasis on conditional]. And De Groot is pointing out that, unlike the other mathematics, one absolute condition in confirmatory statistical analysis is blindness [with the emphasis on absolute]. 

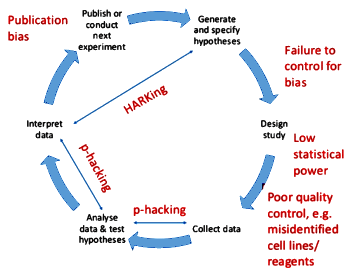

 to metaphorically emphasize the importance of their article, which introduced subpoenaed internal corporate documents to illustrate the fraudulent underbelly of the 2004 Celexa RCT in adolescents. Well, I need an even greater superlative for the De Groot article Dorothy Bishop brings to us from a more naive time – how about the Hope Diamond? Since you’re unlikely to read the whole paper without a nudge, here’s its essence from the translator’s note in the Appendix:
to metaphorically emphasize the importance of their article, which introduced subpoenaed internal corporate documents to illustrate the fraudulent underbelly of the 2004 Celexa RCT in adolescents. Well, I need an even greater superlative for the De Groot article Dorothy Bishop brings to us from a more naive time – how about the Hope Diamond? Since you’re unlikely to read the whole paper without a nudge, here’s its essence from the translator’s note in the Appendix: When I retired a lot of people kept asking me what I was going to do. Their questions made me aware that I had no idea, so I made up something. "I want to find my inner boredom" I would say. Later, I found a more accurate answer, "I want to think about what I want to think about." What I meant was that in the busy·ness of practicing and teaching and the many other things on my plate, my mind was not my own. It was filled with things I needed to attend to, and I wanted it back. I wanted to pick my own topics.
When I retired a lot of people kept asking me what I was going to do. Their questions made me aware that I had no idea, so I made up something. "I want to find my inner boredom" I would say. Later, I found a more accurate answer, "I want to think about what I want to think about." What I meant was that in the busy·ness of practicing and teaching and the many other things on my plate, my mind was not my own. It was filled with things I needed to attend to, and I wanted it back. I wanted to pick my own topics. Saturday, out of the blue, I posted a couple of jazz classics from 1956 [
Saturday, out of the blue, I posted a couple of jazz classics from 1956 [