While it is not fashionable to talk of psychoanalysis or other mind theories these days, I can think of no other way to approach this editorial by the editor of the New England Journal supporting Robert Califf’s nomination for the leadership of the FDA. And the particular mind theory isn’t really psychoanalytic, it’s just what’s called common sense – people do the same things over and over. Freud called it the repetition compulsion and tried to explain it in a variety of ways, but his speculations are immaterial. It’s the phenomenon that matters. If you read this blog frequently, or even occasionally, you can predict what I’m going to write about and what I’m going to say with surprising accuracy. Likewise, you can look at the name of a frequent commenter and predict their response. Watching the presidential debates, you can do the same thing for the candidates you know [and you’ll be able to do it for the ones you don’t know by the next debate]. We call that character.
The word character can be used in a number of contexts. In the moral sphere, it means that a person who has been principled in the past will be principled in the future [predictable repetition]. In drama, the predictable repetitions of the characters are what drive the plot. When you say "He’s a real character," you’re referring to a person with unusual predictable repetitions. But in a psychotherapeutic context, it simply means the individual collection of predictable repetitions, no matter what the context. For a trivial example, when I write these blogs, I invariably use colors, bolds, italics to emphasize certain words. I know it’s kind of annoying, but try as I might, the habit [a predictable repetition] persists. Th ese character traits we all have are enduring, and changing them, even if you know what they are, is a real undertaking. It’s the stuff of psychotherapy, and it’s plenty hard work. It’s not hard to identify maladaptive character traits in others. It easy to see how they account for a person’s own difficulty in negotiating life happily. But there’s nothing slightly easy about helping a person change them – the wisdom of "a leopard can’t change his spots."
ese character traits we all have are enduring, and changing them, even if you know what they are, is a real undertaking. It’s the stuff of psychotherapy, and it’s plenty hard work. It’s not hard to identify maladaptive character traits in others. It easy to see how they account for a person’s own difficulty in negotiating life happily. But there’s nothing slightly easy about helping a person change them – the wisdom of "a leopard can’t change his spots."
Dr. Jeffrey Drazen’s appointment to editor of the NEJM was characterized by intense debates about the whole question of Conflicts of Interest. I wrote about them around his recent play to lighten the NEJM policy for review articles [
a contrarian frame of mind…,
wtf?…,
wtf? for real…,
a narrative…,
not so proud…]. This is just one reminder among many of that episode when he was appointed:
New York Times
By LAWRENCE K. ALTMAN
May 12, 2000
…Dr. Drazen, 53, helped pioneer asthma drugs now taken by four million asthmatics worldwide. In today’s news conference, he strongly defended the need for doctors to work closely and carefully with the drug industry. He called the industry a powerful force without which basic research findings made through taxpayer grants from the National Institutes of Health could not be converted into new therapies to improve patient care and public health. Last February, after an internal investigation prompted by articles in The Los Angeles Times, The Journal found that it had violated its own rules in publishing 19 articles by Dr. Drazen and other authors with industry ties. The Journal said the articles should have been written by scientists without such connections, but its editors blamed themselves and said Dr. Drazen had disclosed his industry support.
Asked today at the news conference about that episode, Dr. Drazen said that as The Journal’s new editor in chief he would hand over all manuscripts dealing with his specialty or products made by the nine companies to deputies "and make sure that they are on the agenda at a time when I do not come to the editorial meeting." In such cases, Dr. Drazen said he wanted "The Journal to be able to judge the science that comes in, if it is good or bad, without me having anything to do with it."
"I do not want to influence things in either a positive or a negative way,” he said. ”We want the good science and good information to get out there” in The Journal, which is one of the most influential in the world. Dr. Drazen, who will leave his Harvard post, will be the Journal’s third editor in chief in less than a year. His selection follows several years of turmoil between the editors of The Journal and its owner, the Massachusetts Medical Society, concerning the society’s increasing business ventures…
I don’t question Dr. Drazen’s character in the moral context – nor, for that matter, Dr. Califf’s. I would expect that they are decent human beings who pay their taxes, don’t text while they drive, and don’t litter. But I do question their predictable repetitions. Dr. Drazen’s appointment was questioned because of his consistent bias in favor of business and industry. He assured us that it would not affect his editorship. And yet here we are fifteen years later and he’s making a major assault on the NEJM’s [exempary] COI policy. And now he’s using his pulpit as NEJM Editor to support a nominee for head of the FDA who is similarly afflicted with industry bias. Like I said, predictable repetitions…
New England Journal of Medicine
by Jeffrey M. Drazen, M.D.
October 28, 2015
Robert M. Califf, M.D., has been nominated to be the next head of the Food and Drug Administration (FDA); he currently serves as Deputy Commissioner for the Office of Medical Products and Tobacco. We think his confirmation as commissioner should proceed as quickly as possible. Because the FDA oversees the safety and, in some spheres, the efficacy of products that constitute about 25% of our economy, the country needs a strong and experienced leader who can keep the FDA focused on its mission.
Since Califf was nominated to succeed Margaret Hamburg, numerous individuals and groups have endorsed his candidacy. His noted strengths include his experience in the testing of new and established drugs for efficacy; his successful career at Duke University, where he was the founding director of the Duke Clinical Research Institute, by many measures one of the premier academic research organizations in the world; and until his FDA nomination, his tenure as head of the Duke Translational Medicine Institute and professor of medicine at Duke University. Over his 30-year academic career, he has published more than 1200 peer-reviewed publications, work he has authored has been cited over 50,000 times, and his Web of Science h-index is 118. But academic output has not been his primary goal; instead, he has worked to accrue the data needed to improve patient care. Despite this laudable aim, a few concerns have been expressed about his associations with industry, and these concerns may have caused some to withhold support for his nomination.
Like Califf, we believe that our actions should be driven by data, not innuendo. Since 2005, Califf has reported, as an investigator, the outcomes of seven clinical trials sponsored solely by industry in primary publications in major general medical journals. Of these trials, four had a negative outcome [i.e., not favoring the intervention], two favored the intervention, and one, with a factorial design, had a mixed outcome. Given this performance, it is impossible to argue that Califf has a pro-industry bias. On top of this, for the past 3 years the vast majority of his funded salary came from leadership roles in the Clinical Translational Science Award from the National Institutes of Health [translational medicine], the NIH Collaboratory, the Patient-Centered Outcomes Research Network [large-scale population health research], and the Duke Center for Medicare and Medicaid Innovation [CMMI] project, which developed a model approach to health care disparities in diabetes, using geospatial mapping to deliver clinical care and social support more effectively.
Our association with Califf grows from a decade of mutual service on the Forum on Drug Discovery, Development, and Translation of the Institute of Medicine [now the National Academy of Medicine]. Through this decade of service, Califf’s primary interest was clearly in gathering and using solid information to promote the health and well-being of people suffering from disease. His aim was always to find better ways to diagnose and treat illness. He wanted well-gathered data on which to base all our clinical decisions and wanted to design and implement health systems that worked effectively to improve the outcomes of individuals and populations. Califf’s experience, his proven leadership abilities, his record of robust research to guide clinical practice, and his unwavering dedication to improving patient outcomes are unsurpassed qualifications for the post of commissioner of the FDA; we strongly endorse his nomination and urge the Senate to act favorably on it.
Drazen says of Dr. Califf, "…it is impossible to argue that Califf has a pro-industry bias." I would conclude the opposite, that it’s impossible to reach any other conclusion. My own bias notwithstanding, I hardly think this is a good time to have a pro-industry biased head of the FDA, no matter how accomplished or moral. Neither is it a time to have a leader with an anti-industry bias. We need a just plain old scientist with no record of predictable repetitions in this area – that’s called common sense…




 Insel is moving from the government to Silicon Valley, he told me last week, because he sees the tech sector as the answer to detecting, diagnosing, and treating mental illness. “Technology can have greater impact on mental healthcare than on the care for heart disease, diabetes, cancer or other diseases,” he said in an interview at Chicago Ideas Week. “It could transform this area in the next five years.”
Insel is moving from the government to Silicon Valley, he told me last week, because he sees the tech sector as the answer to detecting, diagnosing, and treating mental illness. “Technology can have greater impact on mental healthcare than on the care for heart disease, diabetes, cancer or other diseases,” he said in an interview at Chicago Ideas Week. “It could transform this area in the next five years.” He imagines creating “sensors that give you very objective measures of your behavior.” “We do that already for how many steps you’ve had and your activity,” he said, pointing to the Fitbit strapped to his wrist, “but this would be doing it for mood, for cognition, for anxiety. It’s really actually very doable.” The sensors would measure sleep, movement, and have you take clinical tests in order to measure mental health. Other tools could analyze someone’s language use for early signs of psychosis.
He imagines creating “sensors that give you very objective measures of your behavior.” “We do that already for how many steps you’ve had and your activity,” he said, pointing to the Fitbit strapped to his wrist, “but this would be doing it for mood, for cognition, for anxiety. It’s really actually very doable.” The sensors would measure sleep, movement, and have you take clinical tests in order to measure mental health. Other tools could analyze someone’s language use for early signs of psychosis. But the Spire didn’t accurately read my moods, despite its accurate readings of my physical state. At one point while wearing the Spire, I had to do something that made me anxious. Despite my stress, the Spire claimed I was “focused”–most likely because I was forcing myself to concentrate and breathe slowly. My mental state did not match my physical one.
But the Spire didn’t accurately read my moods, despite its accurate readings of my physical state. At one point while wearing the Spire, I had to do something that made me anxious. Despite my stress, the Spire claimed I was “focused”–most likely because I was forcing myself to concentrate and breathe slowly. My mental state did not match my physical one.
 ese character traits we all have are enduring, and changing them, even if you know what they are, is a real undertaking. It’s the stuff of psychotherapy, and it’s plenty hard work. It’s not hard to identify maladaptive character traits in others. It easy to see how they account for a person’s own difficulty in negotiating life happily. But there’s nothing slightly easy about helping a person change them – the wisdom of "a leopard can’t change his spots."
ese character traits we all have are enduring, and changing them, even if you know what they are, is a real undertaking. It’s the stuff of psychotherapy, and it’s plenty hard work. It’s not hard to identify maladaptive character traits in others. It easy to see how they account for a person’s own difficulty in negotiating life happily. But there’s nothing slightly easy about helping a person change them – the wisdom of "a leopard can’t change his spots."  I have no real information about Ketamine as a treatment for depression, though it does seem beyond peculiar to be writing about a club drug in the context of treating a mental illness. But I’m writing about it for another reason nonetheless – a ethical reason. As common as it has become, I protest peer reviewed academic journals publishing articles that are primarily commercials. And my protest extends to publishing articles written by people with strong financial ties to the treatment under discussion in the article. It would be fine with me if there were a specific journal for that kind of paper – the Journal of Industry Financed Reviews and Clinical Trials in Psychopharmacology. I expect there already are a few [or some unacknowledged candidates].
I have no real information about Ketamine as a treatment for depression, though it does seem beyond peculiar to be writing about a club drug in the context of treating a mental illness. But I’m writing about it for another reason nonetheless – a ethical reason. As common as it has become, I protest peer reviewed academic journals publishing articles that are primarily commercials. And my protest extends to publishing articles written by people with strong financial ties to the treatment under discussion in the article. It would be fine with me if there were a specific journal for that kind of paper – the Journal of Industry Financed Reviews and Clinical Trials in Psychopharmacology. I expect there already are a few [or some unacknowledged candidates].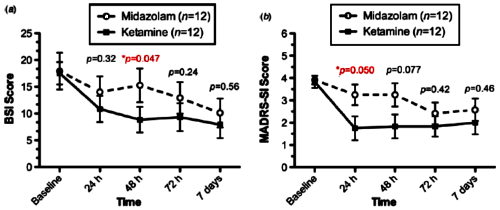
 It appears to me that an academic appointment is generally a requirement to publish an article in a peer reviewed academic journal [there’s good reason for that]. I’ve called it a ticket, one that has, in my opinion, been heavily abused – bought and sold way too frequently. An academic appointment isn’t intended to be a commercial commodity.
It appears to me that an academic appointment is generally a requirement to publish an article in a peer reviewed academic journal [there’s good reason for that]. I’ve called it a ticket, one that has, in my opinion, been heavily abused – bought and sold way too frequently. An academic appointment isn’t intended to be a commercial commodity. On has to be awed by blogger Neuroskeptic whose scope far exceeds the rest of us combined. Here, he clears up the dilemma I obsessed about recently. When the results of the RAISE study were published a few days ago, the extensive press coverage proclaimed that the NAVIGATE [NAV] patients received a much lower dose of antipsychotics than those with treatment as usual [TAU], yet the paper itself said nothing about the drug doses. That’s a hot issue at the moment. Traditional psychiatric teaching has been that maintenance medication [and plenty of it] was an important ongoing part of treatment to prevent psychotic relapses, whereas an initiative from Mad in America, the British Psychological Society, the studies of Wunderink and Harrow, etc. suggest that maintenance medication interferes with long term functional recovery. And the ideological and guild-driven overlay on this point hangs heavily over any and all opinions on this matter. So the difference between the media hype and the actual papers caught the eye of many [including yours truly – see
On has to be awed by blogger Neuroskeptic whose scope far exceeds the rest of us combined. Here, he clears up the dilemma I obsessed about recently. When the results of the RAISE study were published a few days ago, the extensive press coverage proclaimed that the NAVIGATE [NAV] patients received a much lower dose of antipsychotics than those with treatment as usual [TAU], yet the paper itself said nothing about the drug doses. That’s a hot issue at the moment. Traditional psychiatric teaching has been that maintenance medication [and plenty of it] was an important ongoing part of treatment to prevent psychotic relapses, whereas an initiative from Mad in America, the British Psychological Society, the studies of Wunderink and Harrow, etc. suggest that maintenance medication interferes with long term functional recovery. And the ideological and guild-driven overlay on this point hangs heavily over any and all opinions on this matter. So the difference between the media hype and the actual papers caught the eye of many [including yours truly – see  I’m not going to wind up on telepsychiatry today. I complain about enough things already. I think it’s a creation of some cost-cutting bean counter and ought to be outlawed as de facto malpractice rather than the subject of glowing articles in the Psychiatric News. I doubt I even have to explain why I think that, so I won’t. This is hardly my first encounter with such a case, and I’m sure it won’t be my last. Some truths are self-evident…
I’m not going to wind up on telepsychiatry today. I complain about enough things already. I think it’s a creation of some cost-cutting bean counter and ought to be outlawed as de facto malpractice rather than the subject of glowing articles in the Psychiatric News. I doubt I even have to explain why I think that, so I won’t. This is hardly my first encounter with such a case, and I’m sure it won’t be my last. Some truths are self-evident…  I didn’t pursue a Child and Adolescent fellowship, but would’ve if I hadn’t used up my be-a-student quota on a couple of residencies and analytic training. But that idea of developmental deviation stayed with me. Years later I was working in a Child and Adolescent clinic and saw how important that concept really is. Most of the adolescents were brought because of behavioral problem or depressive symptoms and the main thrust of our work with the kids and their families was to help them get back to the business of developing an identity suitable for adult challenges.
I didn’t pursue a Child and Adolescent fellowship, but would’ve if I hadn’t used up my be-a-student quota on a couple of residencies and analytic training. But that idea of developmental deviation stayed with me. Years later I was working in a Child and Adolescent clinic and saw how important that concept really is. Most of the adolescents were brought because of behavioral problem or depressive symptoms and the main thrust of our work with the kids and their families was to help them get back to the business of developing an identity suitable for adult challenges.  When I woke·up after retiring and found how much the academic-industrial alliance had fueled something of a medication-mania, I was most surprised about how it had its fingers in the C&A literature and practice. The Bieder·mania epidemic of the Bipolar Child, the general use of the atypical antipsychotics in disruptive and developmentally challenged children, and the continued use of the SSRI andidepressants in adolescents in spite of the weak to negative clinical trials and Black Box Warning were unwelcome surprises. It was if the distinction between child and adult psychiatry, between children and adults, had eroded and the kids were being treated as little adults – reminiscent of the adultomorphic children of previous centuries.
When I woke·up after retiring and found how much the academic-industrial alliance had fueled something of a medication-mania, I was most surprised about how it had its fingers in the C&A literature and practice. The Bieder·mania epidemic of the Bipolar Child, the general use of the atypical antipsychotics in disruptive and developmentally challenged children, and the continued use of the SSRI andidepressants in adolescents in spite of the weak to negative clinical trials and Black Box Warning were unwelcome surprises. It was if the distinction between child and adult psychiatry, between children and adults, had eroded and the kids were being treated as little adults – reminiscent of the adultomorphic children of previous centuries.