Looking through the December American Journal of Psychiatry, there was a small study in the Letters section, Effect of a Novel NMDA Receptor Modulator, Rapastinel [Formerly GLYX-13], in OCD: Proof of Concept. They had given intravenous Rapastinel [GLYX-13] an NMDA receptor partial blocker to seven patients with severe OCD. Remember Rapastinel [GLYX-13]? It’s the Ketamine-like drug touted to have Ketamine’s short-term antidepressant effect without any of the Psychodelic "Club Drug" effect. In this series, Rapastinel did have an acute effect on the OCD Sx, but unlike in depression, at one week the effect was gone. But enough about that. This Letter just reminded me that I had unfinished business with Rapastinel.
A year ago, the American Psychiatric Association Council on Research published a review article with senior author Dr. Charlie Nemeroff:
by D. Jeffrey Newport, Linda L. Carpenter, William M. McDonald, James B. Potash, Mauricio Tohen, and Charles B. Nemeroff, The APA Council of Research Task Force on Novel Biomarkers and Treatments
American Journal of Psychiatry. 2015 172:950–966.
I wrote about it in [a touch of paralysis…]. Dr. Nemeroff had already gone out of his way to say he was not connected with Naurex, the company behind GLYX-13 [Rapastinel] here, [between 5:40 and 6:40]. That APA review article was enthusiastic about Rapastinel, in spite of the fact that there was only one trial [Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent][NCT01234558] with all authors being involved with Naurex [founded by Joseph Moskal, PhD]. Sure enough, shortly after that review article came out, Allergan bought Naurex for $560 M [Dr. Nemeroff is definitely financially involved with Allergan]. Positively reviewing drugs without acknowledging his own COI was Dr. Nemeroff’s M.O. in the past [see supplement·a·tion: a strange kind of sense…], and frankly I suspected he might be up to his old tricks again – though in a more untouchable way. So I flagged Rapastinel as a trial to take a look at. As I said, the letter in the American Journal of Psychiatry this month just reminded me.
Dr. Moskal and his colleagues have certainly put in the time with their GLIX-13 NMDA partial agonist [now Rapastinel]. He is a neurobiologist at Northwestern [see Northwestern’s $560 million man] who tested his molecule in a wide range of animal models. His articles about GLIX-13 are worth looking over [at least the titles], as he predicts from his basic research many possible clinical applications. But these two titles state clearly where the eagle landed – GLYX-13, an NMDA receptor glycine site functional partial agonist enhances cognition and produces antidepressant effects without the psychotomimetic side effects of NMDA receptor antagonists and GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects [both available on-line].
While there is a gaggle of
basic science articles in the literatiure, there are only a few pertinent
Clinical Trials registered on
ClinicalTrials.gov [none with Results posted]…
…only one of which has been published other that this month’s OCD Letter to the Editor [at least this is the only one I could find]. :
by Preskorn S, Macaluso M, Mehra DO, Zammit G, Moskal JR, Burch RM; and GLYX-13 Clinical Study Group.
Journal of Psychiatric Practice. 2015 21[2]:140-149.
BACKGROUND: … Studies have demonstrated that high affinity N-methyl-Daspartate [NMDA] receptor blockers [eg, ketamine] can produce rapid antidepressant effects in patients who have not responded to currently available agents, but treatment with these agents is accompanied by psychotomimetic effects that make their use problematic. This column describes a POC study involving GLYX-13, an N-methyl-D-aspartate receptor glycine site functional partial agonist.
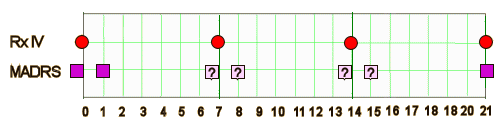
METHOD: In this double-blind, randomized, placebo-controlled study, a single intravenous [IV] dose of GLYX-13 [1, 5, 10, or 30 mg/kg] or placebo was administered to 116 subjects with MDD who had not benefitted from a trial of at least one biogenic amine antidepressant during the current episode. The primary outcome measure was score on the Hamilton Depression Rating Scale-17 [Ham-D17], which was used to rate overall depressive symptoms at baseline and at 24 hours and days 3, 7, 14, and, in some arms, days 21 and 28 after administration.
RESULTS: GLYX-13, 5 or 10 mg/kg IV, reduced depressive symptoms as assessed by the Ham-D17 at days 1 through 7. Onset of action as assessed using the Bech-6 occurred within 2 hours. GLYX-13 did not elicit psychotomimetic or other significant side effects.
CONCLUSION: In this early POC study, GLYX-13 reduced depressive symptoms within 2 hours and this effect was maintained for 7 days on average in subjects with MDD who had not responded to another antidepressant agent during the current depressive episode. The findings of this study support the hypothesis that modulation of the NMDA receptor is a valid target for the development of antidepressant drugs and the need for additional studies to further evaluate the effects of GLYX-13. POC studies such as the one described here play a pivotal role in allowing drug researchers to decide whether to move forward with larger and more expensive studies, and they enable them to focus available resources on those molecules that appear to have the most therapeutic promise. Based on the POC study described here, a multiple dose study has been completed which showed sustained therapeutic benefit with repeated dosing of GLYX-13 for more than 6 weeks. Phase 3 studies are now being planned.
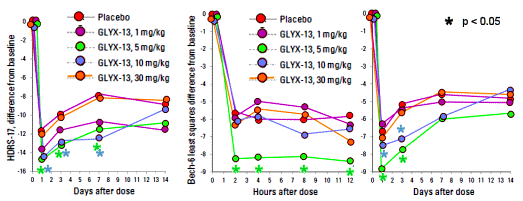 [redrawn for clarity]
[redrawn for clarity]This study was completed in July 2012 but not published until March 2015. A month later, the Nemeroff review article came out, and several months after that, Allergan bought Naurex for $560 M. Their abstract is skimpy on details so I’ve included snippets from the full text. To be honest, the text wasn’t so very forthcoming either. Expected data tables weren’t in attendance – just the graphs:
There was a substantial placebo response in this study with subjects in the placebo group showing an average 45% reduction in Ham-D17 scores at 24 hours post dose…
The subjects who received 1 mg/kg of GLYX-13 had numerically but not statistically sig- nificantly greater reductions in mean Ham-D17 scores compared to those who received placebo. However, the subjects who received 5 and 10 mg/kg of GLYX-13 showed reductions in mean Ham-D17 scores that were both numerically and statistically significantly greater than those that occurred in the placebo group [p < 0.05 for the ANCOVA drug effect of 5 and 10 mg/kg]. While the effect of GLYX-13 differed significantly from placebo at Day 7 after dose administration, the difference between the effect of GLYX-13 and placebo was not significant at Day 14. Differences from placebo on the Ham-D17 ranged from -2.8 units for 5 mg/kg and -2.5 units for 10 mg/kg at day 1 after dosing to -3.1 to for 5 mg/kg and -4.3 units for 10 mg/kg at day 7 following dosing. The effect sizes [Cohen’s ds] at days 1, 3 and 7 were 0.56, 0.41, and 0.39 for the 5 mg/kg dose of GLYX-13 and 0.37, 0.36, 0.60 for the 10 mg/kg of GLYX-13. However, when the dose of GLYX-13 was raised to 30 mg/kg, there was no antidepressant effect… None of the response or remission percentages in subjects receiving GLYX-13 was statistically significant compared to placebo, due to the large placebo effect observed during the first 24 hours after dosing…
And this comment from the discussion was perplexing…
Since this POC was done, a multiple dose study has been recently completed. That study demonstrated sustained antidepressant efficacy in patients whose depressive episode had not been responsive to available biogenic amine antidepressants. Based on the results of this POC study and the subsequent multiple dose study, GLYX-13 is entering phase 3 development…
…because
I couldn’t find any such multiple dose study, either registered on ClinicalTrials.gov or published in PubMed. I’m not saying it doesn’t exist, but it has certainly eluded me [if you know of it, let me know]. This is what they had to say about this trial in
Ketamine and Other NMDA Antagonists…:
… The literature search identified one randomized clinical trial of rapastinel, evaluating the anti-depressant efficacy of a single intravenous infusion at four doses [1,5,10, and 30 mg/kg] with treatment effects assessed at 2, 4, 8, and 12 hours and 1, 3, 7, and 14 days postinfusion.
No statistically significant differences were observed in rates of treatment response or symptom remission associated with placebo [64% and 42%, respectively] versus rapastinel at any dose [up to 70% and 53%, respectively]. However, statistically significant differences in the reduction of the 17-item HAM-D scores were observed for the 5-mg/kg dose at all intervals except day 14 [peak 17-itcm HAM-D reduction 3.1 points greater than placebo] and the 10-mg/kg dose at day 1 and day 3 [peak 17-itcm HAM-D reduction 4.3 points greater than placebo]. Neither the low [1 mg/kg] nor high [30 mg/kg] rapastinel doses were associated with significant greater 17-item HAM-D score reduction than placebo, leading the authors to posit an inverted U-shape dose response curve.
Psychotomimetic and dissociative side effects. The BPRS positive symptom subscale was administered in this trial with no differences observed between the placebo group and any of the four rapastinel treatment groups. The Clinician-Administered Dissociative States Scale was not administered in this study.
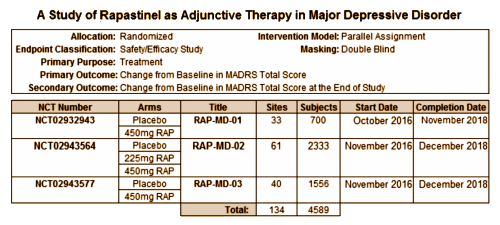
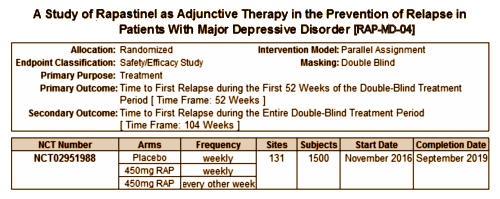
It’s hard for me to believe that Allergan would pay that kind of money based on just one Clinical Trial., but, looking at Clinical Trials.gov, they’ve already registered and started four of their own Phase III Clinical Trials – all focused on Rapastinel as an Adjunctive Therapy in MDD cases:
There are adverse effects with all psychiatric drugs, but we’ve traditionally reserved the more radical empirical treatments for patients who are desperately ill – ECT, antipsychotics, etc. The patients involved in the study reviewed here have a made-up version [Treatment Resistant Depression] of a made-up clinical entity [Major Depressive Disorder].
"Subjects were recruited from investigator practices and via newspaper, radio and Internet advertisements."
They don’t strike me as desperately ill and for that reason, I’ve personally recoiled from the Ketamine fervor altogether. A weekly intravenous injection of a hallucinogenic drug feels like falling prey to therapeutic zeal, one of the traditionally great dangers in medical practice [along with the other head of that dragon – commercial zeal]. But here we are being offered a partial solution to my objection. It’s still an intravenous drug, but at least it’s not heir to Timothy Leary’s fifty year old injunction "
turn on, tune in, drop out." GLYX-13 is being advertised as a non-psychedelic version of Ketamine that has its antidepressant properties [they were working on an oral version –
NRX-1074 [
AGN-241660]. But this blog isn’t really focused on my opinions as an aging psychotherapist [though I certainly have opinions]. It’s about the scientific malfeasance that has bombarded psychiatric medication reporting for the last thirty years. The promise offered by this drug is obvious, but there’s a lot here that deserves a good dose of skepticism…
-
This is an unreplicated lone study of a drug [financed with venture capital].
-
All authors have loud conflicts of interest.
-
There are no "Results" posted on clinicaltrials.gov even though it was completed 4½ years ago.
-
The expected Mean and Standard Deviation data for the raw HAM-D outcome variable by dose by time isn’t in the paper. All we see are the point differences in a graph [with no way to assess variance]. It can’t be vetted…
-
There is a very peculiar plummet in depression scores two hours after the injection and their speculations about "the why?" just don’t pass muster. This drug isn’t supposed to have a "druggie" effect, but it sure seems to have something that smells suspiciously like that!
-
There are at least two other GLYX-13 studies mentioned, but nowhere found eg the Clinical Trial registered as
NCT01684163 completed 2½ years ago, and the multiple dose trial mentioned in the text of this paper.
-
The shout-out in an APA AJP Review article has a first author [Newport] with COI with the developing company [Naurex] reporting for a panel lead by a notoriously tarnished KOL [Nemeroff] with COI with the company that just bought the drug [Allergan].
… for starters. So from where I sit, this one deserves our closest scrutiny all along the way. It’s a block-buster-in-training, and our history with such drugs hasn’t made any of us proud…