Suicidal Thoughts and Behavior With Antidepressant Treatment
Reanalysis of the Randomized Placebo-Controlled Studies of Fluoxetine and Venlafaxine
by Robert D. Gibbons, Hendricks Brown, Kwan Hur, John M. Davis, and J. John Mann
Archives of General Psychiatry. Online February 6, 2012.
[full text on-line]
Context: The US Food and Drug Administration issued a black box warning for antidepressants and suicidal thoughts and behavior in children and young adults.
Objective: To determine the short-term safety of antidepressants by standard assessments of suicidal thoughts and behavior in youth, adult, and geriatric populations and the mediating effect of changes in depressive symptoms.
Data Sources: All intent-to-treat person-level longitudinal data of major depressive disorder from 12 adult, 4 geriatric, and 4 youth randomized controlled trials of fluoxetine hydrochloride and 21 adult trials of venlafaxine hydrochloride.
Study Selection: All sponsor-conducted randomized controlled trials of fluoxetine and venlafaxine. Data Extraction: The suicide items from the Children’s Depression Rating Scale–Revised and the Hamilton Depression Rating Scale as well as adverse event reports of suicide attempts and suicide during active treatment were analyzed in 9185 patients (fluoxetine: 2635 adults, 960 geriatric patients, 708 youths; venlafaxine: 2421 adults with immediate-release venlafaxine and 2461 adults with extended-release venlafaxine) for a total of 53 260 person-week observations.
Data Synthesis: Suicidal thoughts and behavior decreased over time for adult and geriatric patients randomized to fluoxetine or venlafaxine compared with placebo, but no differences were found for youths. In adults, reduction in suicide ideation and attempts occurred through a reduction in depressive symptoms. In all age groups, severity of depression improved with medication and was significantly related to suicide ideation or behavior.
Conclusions: Fluoxetine and venlafaxine decreased suicidal thoughts and behavior for adult and geriatric patients. This protective effect is mediated by decreases in depressive symptoms with treatment. For youths, no significant effects of treatment on suicidal thoughts and behavior were found, although depression responded to treatment. No evidence of increased suicide risk was observed in youths receiving active medication. To our knowledge, this is the first research synthesis of suicidal thoughts and behavior in depressed patients treated with antidepressants that examined the mediating role of depressive symptoms using complete longitudinal person level data from a large set of published and unpublished studies.
-
Study Evaluating Desvenlafaxine Succinate Sustained-Release [DVS SR] In The Treatment Of Child And Adolescent Outpatients With Major Depressive Disorder
COMPLETED RESULTS
Major Depressive Disorder
Desvenlafaxine Succinate Sustained-Release Tablets [DVS SR]
Wyeth is now a wholly owned subsidiary of Pfizer
February 2008-November 2009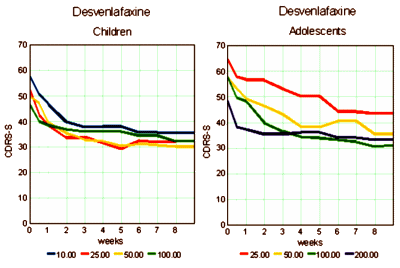
-
Study Evaluating Desvenlafaxine Succinate Sustained-Release Tablets [DVS SR] In The Treatment Of Child and Adolescent Outpatients With Major Depressive Disorder
COMPLETED RESULTS C-SSRS
Major Depressive Disorder
Desvenlafaxine Succinate Sustained-Release Tablets [DVS SR]
Wyeth is now a wholly owned subsidiary of Pfizer
May 2008-May 2010 -
A Study Of DVS SR In Treatment Of Children And Adolescent Outpatients With MDD
RECRUITING
Major Depressive Disorder
Desvenlafaxine Succinate Sustained-Release; Placebo
Pfizer
August 2011-August 2015 -
A 6-Month Open-Label Extension Study to the B2061014 Study to Evaluate the Safety, Tolerability and Efficacy of DVS SR in the Treatment of Children and Adolescents With MDD
RECRUITING
Major Depressive Disorder
DVS SR
Pfizer
February 2012-March 2016 -
A 6-Month Extension Study To The B2061032 Study To Evaluate The Safety, Tolerability, And Efficacy Of DVS SR In The Treatment Of Child And Adolescent Outpatients With MDD
RECRUITING
Major Depressive Disorder
DVS SR
Pfizer
January 2012-February 2016 -
A Study Of DVS SR In Treatment Of Children And Adolescent Outpatients With MDD
RECRUITING
Major Depressive Disorder
desvenlafaxine succinate sustained release; fluoxetine; placebo
Pfizer
November 2011-August 2015
-
A Study in the Treatment of Children and Adolescents With Major Depressive Disorder
COMPLETED
Major Depressive Disorder
Placebo; fluoxetine; duloxetine
Eli Lilly and Company
March 2009-September 2011 -
Open-Label Study of Duloxetine in the Treatment of Children and Adolescents With Major Depressive Disorder
COMPLETED RESULTS C-SSRS
Major Depressive Disorder
duloxetine
Eli Lilly and Company
August 2007-September 2008 -
A Study in the Treatment of Children and Adolescents With Major Depressive Disorder
COMPLETED
Major Depressive Disorder
duloxetine; Placebo; fluoxetine
Eli Lilly and Company
March 2009-October 2011
The Columbia–Suicide Severity Rating Scale: Initial Validity and Internal Consistency Findings From Three Multisite Studies With Adolescents and Adults
by Kelly Posner, Ph.D.; Gregory K. Brown, Ph.D.; Barbara Stanley, Ph.D.; David A. Brent, M.D.; Kseniya V. Yershova, Ph.D.; Maria A. Oquendo, M.D.; Glenn W. Currier, M.D., M.P.H.; Glenn A. Melvin, Ph.D.; Laurence Greenhill, M.D.; Sa Shen, Ph.D.; and J. John Mann, M.D.
American Journal of Psychiatry 2011 168:1266-1277.
Objective: Research on suicide prevention and interventions requires a standard method for assessing both suicidal ideation and behavior to identify those at risk and to track treatment response. The Columbia–Suicide Severity Rating Scale [C-SSRS] was designed to quantify the severity of suicidal ideation and behavior. The authors examined the psychometric properties of the scale.
Method: The CSSR-S’s validity relative to other measures of suicidal ideation and behavior and the internal consistency of its intensity of ideation subscale were analyzed in three multisite studies: a treatment study of adolescent suicide attempters [N=124]; a medication efficacy trial with depressed adolescents [N=312]; and a study of adults presenting to an emergency department for psychiatric reasons [N=237].
Results: The C-SSRS demonstrated good convergent and divergent validity with other multi-informant suicidal ideation and behavior scales and had high sensitivity and specificity for suicidal behavior classifications compared with another behavior scale and an independent suicide evaluation board. Both the ideation and behavior subscales were sensitive to change over time. The intensity of ideation subscale demonstrated moderate to strong internal consistency. In the adolescent suicide attempters study, worst-point lifetime suicidal ideation on the CSSR-S predicted suicide attempts during the study, whereas the Scale for Suicide Ideation did not. Participants with the two highest levels of ideation severity [intent or intent with plan] at baseline had higher odds for attempting suicide during the study.
Conclusions: These findings suggest that the C-SSRS is suitable for assessment of suicidal ideation and behavior in clinical and research settings.
So what is my conclusion? I don’t have one yet, but I have a high level of suspicion that the forces at work here are being driven rather than just serendipity. I’m suspicious that Dr. Gibbons fervor has a cause. I see two drug companies that stand to gain a lot by either pediatric approval or a lifting of the warning. I see a new suicidality scale being introduced and used in these studies, shepherded by the same author writing with Dr. Gibbons. I read the comments of a Biostatistician talking about physician motives and what needs to be done for patients – odd. And given the track record of this industry, in this specialty, in this time, I say that there’s enough here to try to keep this issue on the very front burner. On the other side of the coin, I don’t see a lot of evidence that these drugs are very effective in kids and adolescents in the published studies [tuning the quartet…, clinically insignificant study…] or in my own experience treating kids. Yes, I do use them. Yes I have seen them help, primarily in kids with an obsessive compulsive diathesis, but also at times with depression. But the idea that being careful with antidepressants in kids is depriving them of some kind of vital treatment is patently absurd from where I sit. Their effectiveness is such that one is glad when they do work [some], and hardly surprised when they don’t.
how is it that the “archives of general psychiatry” provides a free full-text view of the gibbons article?
other articles are not accorded this status
is someone paying for this “free full-text view”?
Excellent point! It’s very unusual as best I can tell. I had the same suspicions…
I’ve been reading a lot of your posts. As a person who has been labelled as “bipolar” over a reaction to a drug, who discovered after years of useless and worse polypharmacy that the “depression” I was so afraid of having again was pernicious anemia, it gives me comfort to see someone who understands the numbers to say what I’ve been thinking for a long time.
When I last started feeling unexplained fatigue, I did not go to a psychiatrist and describe myself as depressed and I’m glad of it. That fatigue eventually proved itself to be one of the effects of Multiple Sclerosis. I have no doubt that had I subjected myself to the psychiatric industry I would have been prescribed one cocktail after another and would have been described as “treatment resistant”.
After my traumatic experience in nuclear forces the most twisting and damaging force I’ve dealt with is psychiatry. their labels, their dark prognostications, and their drugs.
I did work out a lot of childhood issues with therapy before therapists started asserting in the first meeting that they wanted to put me on an antidepressant to make therapy “easier”. I find it unaffordable, if not impossible to get any kind of talk therapy that is more about listening than imposing a school of therapeutic tactics. It offends me that so many mental health professionals make so much for doing so little for their patients, especially those with MDs who appear to believe that once a person has been diagnosed with a mental disorder EVERYTHING is in the patient’s head.
Why are the Archives of General Psychiatry and American Journal of Psychiatry etc. publishing articles with unintelligible charts and statistics? Isn’t this something the editorial board is supposed to review?
so what is the job of an editor? Especially for a professional journal?