Posted on Friday 25 December 2015

Well, I can’t seem to let this topic lie. After my last two posts, I ran across a letter to the editor in the American Journal of Psychiatry from Dr. Bernard Carroll, a frequent commenter here, written back in 2009 in response to the meta-analysis by Nelson and Popakostas [Atypical Antipsychotic Augmentation in Major Depressive Disorder: A Meta-Analysis of Placebo-Controlled Randomized Trials]. I’m posting it here with his permission for several reasons. First, he anticipates the cautions expressed in the 2013 meta-analysis by Spielmans et al [Adjunctive Atypical Antipsychotic Treatment for Major Depressive Disorder: A Meta-Analysis of Depression, Quality of Life, and Safety Outcomes] as well as my own concerns about this whole augmentation strategy. In addition, he fleshes out the risk/benefit ratio equation with some actual estimates:
|
Antipsychotic Drugs for Depression?
Bernard J. Carroll, M.B.B.S., Ph.D., F.R.C.Psych. American Journal of Psychiatry. 2010 167[2]:216. |
|
To the Editor: The Review and Overview by J. Craig Nelson, M.D., and George I. Papakostas, M.D., published in the September 2009 issue of the Journal, concluded that atypical antipsychotic drugs "are effective augmentation agents in major depressive disorder but are associated with an increased risk of discontinuation due to adverse events". This meta-analysis did not demonstrate effectiveness, only nominal efficacy against placebo. Moreover, the review is unbalanced because risk was not adequately considered.
The authors called the risk of tardive dyskinesia a "rare but serious" concern. Tardive dyskinesia is serious but not rare with atypical antipsychotic drugs. A rate of 8% has been seen in delusional depression. Many cases are persistent, and depressed patients are especially at risk. For aripiprazole, which was the first atypical antipsychotic drug approved for augmentation in depression, a 1.1% rate of dyskinesia within 6 weeks was reported [study #CN138165; ClinicalTrials.gov registry number, NCT00105196]. This translates to 11,000 cases per million exposures. Should we really give this drug to millions of nonpsychotic depressed patients? The marketer’s aggressive media advertising appears to have that aim. There is insufficient evidence of benefit to offset such risk. The trials of aripiprazole were unblinded by akathisia [25%] and other side effects that can introduce rater bias. Aripiprazole was not efficacious by self-report depression measures, i.e., patients did not find it effective. In one study cited, efficacy was lacking in male subjects. In the meta-analysis, the number needed to treat was nine, signifying only borderline utility in this therapeutic context. The corresponding number needed to treat for lithium is four to five. The larger numbers of patients in the evidence base for atypical antipsychotic drugs, as noted by the authors, reflect the commercial interest in penetrating the depression market, but the manufacturers of these drugs have studiously avoided testing their products against lithium. These concerns about efficacy and relative efficacy were not addressed. Finally, there is no evidence that augmentation with atypical antipsychotic drugs is useful in the long-term. Drs. Nelson and Papakostas did not note that a study of depressive relapse prevention with risperidone augmentation was negative. No long-term safety or efficacy data have been posted for aripiprazole in the treatment of depression, even though a 52-week study was completed in November 2007 [ClinicalTrials.gov registry number, NCT00095745]. On the basis of the presently available data, psychiatrists should be more forthright in pointing out the limited effectiveness as well as the risks and adverse effects of atypical antipsychotic drugs in the treatment of depression, especially in primary care.
|
Continued from a worksheet post…
In April 2015, the FDA approved a new Atypical Antipsychotic, Brexpiprazole [Rexulti®]. Around the same time, there were two articles about the Brexpiprazole clinical trials, both released on-line ahead of print in prominent journals [see the spice must flow…]. But there were some odd things about those articles. Each one had only one academic author – the rest were pharmaceutical company employees. But there was more [see anything goes…] – the academic authors were from the same department of psychiatry, the same institute [Feinstein Institute for Medical Research], with similar conflicts of interest, and the medical ghost-writers were also from the same communications firm [QXV Communications, Macclesfield, U.K.]. These were the studies that the FDA evaluated in the approval process. These articles were virtual clones.
Lundbeck Press ReleaseSeptember 24, 2014
H. Lundbeck A/S [Lundbeck] and Otsuka Pharmaceutical Co., Ltd. [Otsuka] today announced that the US Food and Drug Administration [FDA] has determined that the New Drug Application [NDA] for brexpiprazole for monotherapy in adult patients with schizophrenia and for adjunctive treatment of major depressive disorder [MDD] in adult patients is sufficiently complete to allow for a substantive review and the NDA is considered filed as of 9 September 2014 [60 days after submission]. The PDUFA date is July 11, 2015…
by Thase ME, Youakim JM, Skuban A, Hobart M, Zhang P, McQuade RD, Nyilas M, Carson WH, Sanchez R, and Eriksson H.Journal of Clinical Psychiatry. 2015 76[9]:1232-1240.
by Thase ME, Youakim JM, Skuban A, Hobart M, Augustine C, Zhang P, McQuade RD, Carson WH, Nyilas M, Sanchez R, and Eriksson H.Journal of Clinical Psychiatry. 2015 76[9]:1224-31.

I’m not in love with this story about the marketing practices of big PHARMA, but that’s not really any of my business. But there’s something else that is. When I first started working as a volunteer in the clinics up here, it seemed like everyone was taking Seroquel®. I couldn’t figure out why. At the time, it was the number one selling drug in the country. That’s actually what got me blogging here in the first place. Over time I finally got every non-psychotic patient off of the Seroquel® – worried about Tardive Dyskinesia and the metabolic syndrome, particularly with long term use. I was kind of proud about getting them off that drug. Recently, our clinic has expanded its scope, so there has been an influx of new patients, at least new to me, and guess what? – a lot of them are on Atypical Antipsychotics [not just Seroquel®, but Abilify® and the others]. But here’s the bad part, I’ve got three of those new cases who have definite early Tardive Dyskinesia [a lot for my small practice]. Mercifully, two seem to be waning now they’re off of the drug [fingers crossed], but I’m afraid one is probably around forever. They’ve all been on the drugs for a long time [which is what happens these days]. And I doubt such symptoms happen in short trials.
Although the acute efficacy of the adjunctive atypical antipsychotics is well established, the major shortcoming is the dearth of long-term placebo-controlled studies that would inform clinicians about persistence of efficacy or burden of adverse effects. Only two studies have been performed. In one, risperidone failed to have a significant effect in preventing relapse; however, methodological problems may have contributed to the result. A recent 26-week relapse prevention study of the olanzapine-fluoxetine combination showed a significant advantage for the combination. In that trial, after 6–8 weeks of acute treatment with olanzapine and fluoxetine and 12 weeks of stabilization, patients were randomly assigned to the combination or to fluoxetine plus placebo. A time-to-relapse analysis significantly favored the combination. Under the primary definition of relapse, 15.8% of the patients on the combination relapsed, compared with 31.8% of the patients on fluoxetine. Nevertheless, the paucity of long-term controlled studies for the atypical agents limits our understanding of the persistence of efficacy and of side effects that may increase or emerge with time. This leaves the clinician whose patient has responded to acute adjunctive treatment with little information for weighing the risks and benefits of continuing the adjunctive agent.
So that’s why I liked the Spielmans et al meta-analysis [the extra mile…]. It showed us that from the patients’ own ratings, they weren’t getting that much benefit – a very important piece of data. And for that matter, Spielmans et al documented the Adverse Events including the high incidence of Akasthisia [a Tardive Dyskinesia harbinger] with Abilify®. So I know why I’ve been so stuck on this business of Atypical Antipsychotic augmentation of the so-called treatment-resistant-depression. All of these Atypicals may have a low moderate effect on TRD symptoms, sure enough, but these drugs are time-bombs [particularly] if they are used long term [and they are used long-term!]. So if nobody’s really looked long term in the last 16 years, Why the hell not?
So when I got on my current kick of looking into augmenting SSRI failures with Atypical Antipsychotics, in spite of my skepticism, I wondered "What mechanism they proposed?" and "Who thought up trying it the first time?" Usually, the answers to such questions just come up when I’m darting from article to article – but not this time. So I actually wrote some people. A few laughed and said, "Rationale? They don’t need a Rationale," one adding, "there were drugs to be sold!" Most simply said something like, "No clue." Nobody seemed to know.
by Charles B. Nemeroff, M.D., Ph.D.Journal of Clinical Psychiatry. 2005 66 [suppl 8].
Treatment options for bipolar depression and treatment-resistant unipolar depression include augmentation of antidepressant therapy with a nonantidepressant drug, including atypical antipsychotics. Risperidone is effective in combination with fluvoxamine, paroxetine, or citalopram in treatment-resistant unipolar depression, with reported remission rates of 61% to 76%. Olanzapine in combination with fluoxetine is safe and effective in patients with bipolar depression and those with fluoxetine-resistant unipolar depression. Ziprasidone and aripiprazole augmentation of various selective serotonin reuptake inhibitors has been reported to be effective in refractory unipolar depression in open-label studies. Data on use of quetiapine or clozapine as augmentation therapy for depression or anxiety are not yet available. Further double-blind, placebo-controlled studies of augmentation of antidepressants with atypical antipsychotics in refractory depression and anxiety are justified based on the available literature.
Support for investigation of atypical antipsychotics in patients with depression comes partly from preclinical studies suggesting that several atypical antipsychotics are potent 5-HT2A antagonists at low doses31–33 and may facilitate the action of serotonin at the 5-HT1A receptor, thereby augmenting the efficacy of SSRIs24…
by Robert B. Ostroff, M.D., and J. Craig Nelson, M.D.Journal of Clinical Psychiatry. 1999 60:256–259.
Background: At low doses, risperidone acts as a 5-HT 2 antagonist. Preclinical data suggest 5-HT 2 antagonists may enhance the action of serotonin. This report examines the clinical use of risperidone to augment selective serotonin reuptake inhibitor [SSRI] antidepressants in patients who have not responded to SSRI therapy.Method: In 8 patients with major depressive disorder without psychotic features [DSM-IV] who had not responded to an SSRI, risperidone was added to the ongoing SSRI treatment. Hamilton Rating Scale for Depression scores were obtained before and after the addition of risperidone.Results: These 8 patients remitted within 1 week of the addition of risperidone. Risperidone also appeared to have beneficial effects on sleep disturbance and sexual dysfunction.Conclusion: Risperidone may be a useful adjunct to SSRIs in the treatment of depression.
"The current article describes the use of risperidone as an augmentation strategy. Both preclinical and clinical data provide suggestive evidence that augmentation with risperidone might be effective in treating depression. Risperidone is an atypical antipsychotic, which, at low doses, is about 100 times more potent in antagonizing the 5-HT 2A receptor than the D 2 receptor. The 5-HT 2A receptor is an excitatory receptor that acts in opposition to the postsynaptic 5-HT 1A receptor; thus, antagonism of the 5-HT 2A may facilitate the action of serotonin at the 5-HT 1A receptor. This was demonstrated by a preclinical study [in rats, in 1985] which found that ketanserin, a 5-HT 2 antagonist, enhanced the inhibitory effects of serotonin on prefrontal neurons. This preclinical finding suggests that addition of a 5-HT 2 antagonist to a selective serotonin reuptake inhibitor [SSRI] might augment the effects of the SSRI."
by Lakoski JM, Aghajanian GK.Neuropharmacology. 1985 24:265–273.The ability of the putative serotonin2 [5-HT2] antagonist ketanserin, to alter serotonin [5-HT]-induced responses in cell firing was examined in the prefrontal cortex, the lateral geniculate nucleus and the dorsal raphe nucleus of the rat by microiontophoretic extracellular single unit recording techniques. In the prefrontal cortex, ketanserin failed to antagonize the inhibitory effects of 5-HT recorded in cerveau isolé or preparations anesthetized with chloral hydrate [pure excitatory responses to 5-HT were not observed in either of these preparations]. Paradoxically, the inhibitory response produced by 5-HT [but not gamma-aminobutyric acid, tryptamine or norepinephrine] was potentiated, even in cells where ketanserin alone did not alter spontaneous firing rates. The systemic administration of ketanserin [5 mg/kg, i.p.] had effects similar to those observed in the microiontophoretic experiments in the prefrontal cortex. In the dorsal raphe nucleus of animals anesthetized with chloral hydrate, ketanserin neither attenuated nor potentiated the inhibition of serotonergic neurons by 5-HT. In the lateral geniculate nucleus, as in the prefrontal cortex, ketanserin potentiated rather than attenuated, the inhibitory effect of 5-HT. Ketanserin was found to attenuate the excitatory responses produced by norepinephrine, an alpha 1-adrenoceptor-mediated response, in the lateral geniculate nucleus. The observed potentiation by ketanserin of inhibitory responses to 5-HT but not those of gamma-aminobutyric acid, tryptamine or norepinephrine, recorded in the prefrontal cortex, may be consistent with the proposed interaction between ketanserin and a specific 5-HT2 binding site.

by J. Craig Nelson, M.D. and George I. Papakostas, M.D.American Journal of Psychiatry. 2009 166:980-991.
Objective: The authors sought to determine by meta-analysis the efficacy and tolerability of adjunctive atypical antipsychotic agents in major depressive disorder.Conclusions: Atypical antipsychotics are effective augmentation agents in major depressive disorder but are associated with an increased risk of discontinuation due to adverse events.
by Glen I. Spielmans, Margit I. Berman, Eftihia Linardatos, Nicholas Z. Rosenlicht, Angela Perry, and Alexander C. TsaiPLoS Medicine. 2013 10[3]:e1001403.
Background: Atypical antipsychotic medications are widely prescribed for the adjunctive treatment of depression, yet their total risk–benefit profile is not well understood. We thus conducted a systematic review of the efficacy and safety profiles of atypical antipsychotic medications used for the adjunctive treatment of depression.Conclusions: Atypical antipsychotic medications for the adjunctive treatment of depression are efficacious in reducing observer-rated depressive symptoms, but clinicians should interpret these findings cautiously in light of [1] the small-to-moderate-sized benefits, [2] the lack of benefit with regards to quality of life or functional impairment, and [3] the abundant evidence of potential treatment-related harm.
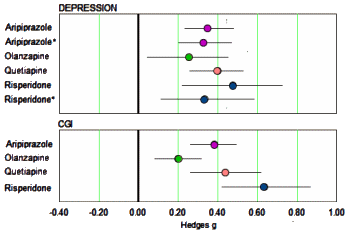
The only continuous self-report measure of depression used in these trials was the Inventory of Depressive Symptomatology Self Report. Continuous measures of quality of life included the Quality of Life Enjoyment and Satisfaction Questionnaire [Q-LES-Q] and the Short Form 36 Health Survey [SF-36]. The only continuous measure of functional impairment employed in these trials was the Sheehan Disability Scale [SDS]…

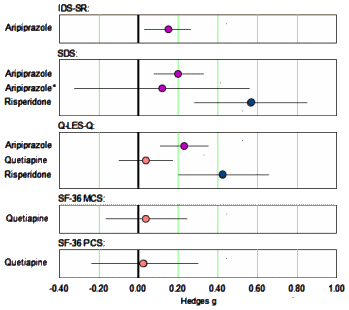
…The effects of risperidone may have been exaggerated by the reliance on post hoc analysis rather than a priori analysis in the largest study of the drug, as the effect of the drug was greater at 6 wk (g= 0.46) than at the prespecified primary end point of 4 wk [g= 0.32].…The effect of aripiprazole on quality of life/functioning should be interpreted with caution, as the effect for the drug on the SDS was very small and no longer statistically significant when patients who violated study protocol were excluded from analysis [g=0.12, p=0.08]. Similarly, the effect of risperidone on quality of life/functioning should be interpreted tentatively since it is largely driven by post hoc analyses.
by Davis CE.American Journal of Epidemiology. 1976 104[5]:493-498.
"Regression to the mean is the phrase used to identify the phenomenon that a variable that is extreme on its first measurement will tend to be closer to the center of the distribution for a later measurement. In studies based on biological measurements, this variability can be attributed to both the inherent variation in the phenomenon being measured and the variability of the measurement itself. The concept of regression to the mean is an important consideration in studies where subjects are chosen because of a biological variable above or below a specified level…
 It’s counter-intuitive. It means if you give a metric like the HAM-D to a group, and then pick out one subject who scores in the shaded area [above a set cut-off value], the subsequent value from that subject will tend to be lower – moving down towards the mean. Likewise, a subject with a score at the lower end’s next value will tend to be higher – again moving up towards the mean. Why don’t they stay the same? Perhaps that’s why regression to the mean is so hard to hold onto, because it’s the essence of what’s different about
It’s counter-intuitive. It means if you give a metric like the HAM-D to a group, and then pick out one subject who scores in the shaded area [above a set cut-off value], the subsequent value from that subject will tend to be lower – moving down towards the mean. Likewise, a subject with a score at the lower end’s next value will tend to be higher – again moving up towards the mean. Why don’t they stay the same? Perhaps that’s why regression to the mean is so hard to hold onto, because it’s the essence of what’s different about 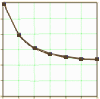 statistics and our everyday dealings with numbers. We’re working with a trend rather than a certainty, with values that move when no operation has been applied except resampling. And since the speed with which repeated samples tend to creep towards the mean slows down as it gets closer, it tends to to create a graph that looks familiar [often misinterpreted]. And when we go hunting for the reasons, we find explanations like this…
statistics and our everyday dealings with numbers. We’re working with a trend rather than a certainty, with values that move when no operation has been applied except resampling. And since the speed with which repeated samples tend to creep towards the mean slows down as it gets closer, it tends to to create a graph that looks familiar [often misinterpreted]. And when we go hunting for the reasons, we find explanations like this…
 … and maybe that’s another reason it doesn’t stick – who’s going to carry that around in their mind? Apparently, not me. Probably you won’t either [if you even read it]. So since what we’re aiming for isn’t the why? of regression to the mean, but the why? it won’t stay in mind, maybe we should come at it from a different angle – base it on something we already know. We all seem to accept that if we repeatedly sample something in nature, we’ll get a range of answers, and if we look at the frequency in that range, it’ll look
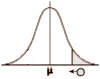
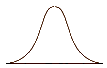
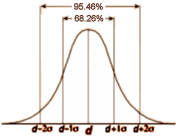
… and maybe that’s another reason it doesn’t stick – who’s going to carry that around in their mind? Apparently, not me. Probably you won’t either [if you even read it]. So since what we’re aiming for isn’t the why? of regression to the mean, but the why? it won’t stay in mind, maybe we should come at it from a different angle – base it on something we already know. We all seem to accept that if we repeatedly sample something in nature, we’ll get a range of answers, and if we look at the frequency in that range, it’ll look 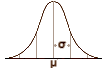 like the curve on the right. Then we can find a Mean [μ] that we accept as the true value [though they’re really all true]. And we can find a Standard Deviation [σ] that represents the variability. We even call it the normal distribution – a testimony to the fact that’s it’s what we normally expect to find in nature.
like the curve on the right. Then we can find a Mean [μ] that we accept as the true value [though they’re really all true]. And we can find a Standard Deviation [σ] that represents the variability. We even call it the normal distribution – a testimony to the fact that’s it’s what we normally expect to find in nature.
If you think about it, the shape of the normal distribution is nothing more than an example of regression to the mean. Our repeated samples move towards and collect around the Mean [μ] as if pulled there by some invisible force – a gravity [but as Einstein pointed out about Newton’s force of gravity, it’s not really a force – it’s just in the nature of things]. So regression to the mean is simply a part of sampling nature. And why? should we expect that some extreme value would do anything but trend towards the mean of the distribution it’s part of? Duh! It’s trying to go towards home or maybe home is calling [if you go for anthropomorphic metaphors].
Probably a better way to think about why? we forget about regression to the mean, or why? we expect that some extreme value would do anything but migrate toward the mean, would be to think about our thinker. We invented a mathematics of arithmetic and algebra to fit our minds, so we’re the exceptions. And we try to pull nature into the way our minds work. It’s a bit like our building things based on straight lines and right angles, whereas nature builds with an infinite series of curves [the beauty of nature we so admire]. What we call statistics is our attempt to see things as distributions rather than singularities, as trends rather than certainties, and it’s often hard going because the mind keeps trying to pull things back into our right-angle-straight-line frameworks [more anthropomorphism]. But what I call the land of sometimes is actually closer to the real world of nature than our precision mathematics.
Clinical Outcome After Antipsychotic Treatment Discontinuation in Functionally Recovered First-Episode Nonaffective Psychosis Individuals: A 3-Year Naturalistic Follow-Up Studyby Jacqueline Mayoral-van Son, MD; Victor Ortiz-Garcia de la Foz, VTE; Obdulia Martinez-Garcia, PhD; Teresa Moreno, MD; Maria Parrilla-Escobar, MD; Elsa M. Valdizan, MD, PhDc; and Benedicto Crespo-Facorro, MD, PhDJournal of Clinical Psychiatry. Published online 12/08/2015
Objective: The timing of antipsychotic discontinuation in patients who have fully recovered from their initial episode of psychosis is still open to discussion. We aimed to evaluate the risk of symptom recurrence during the 3 years after antipsychotic discontinuation in a sample of functionally recovered first-episode nonaffective psychosis [FEP] patients [DSM-IV criteria] with schizophrenia spectrum disorder.Method: Participants in this open-label, nonrandomized, prospective study were drawn from an ongoing longitudinal intervention program of FEP from a university hospital setting in Spain. From July 2004 to February 2011, functionally recovered FEP individuals were eligible if they met the inclusion criteria of [1] a minimum of 18 months on antipsychotic treatment, [2] clinical remission for at least 12 months, [3] functional recovery for at least 6 months, and [4] stabilization at the lowest effective doses for at least 3 months. Forty-six individuals who were willing to discontinue medication were included in the discontinuation group [target group]. Twenty-two individuals opted to stay on the prescribed antipsychotic medication and therefore were included in the maintenance group [control group]. Primary outcome measures were relapse rate at 18 and 36 months and time to relapse.Results: The rates of relapse over the 3-year period were 67.4% [31 of 46] in the discontinuation group and 31.8% [7 of 22] in the maintenance group. The mean time to relapse was 209 [median = 122] days and 608 [median = 607] days, respectively [log rank = 10.106, P = .001]. The resumption of antipsychotic medication after the relapse occurred was associated with clinical stability and lack of further relapses. When the overall group of relapsed individuals from the 2 conditions [N = 38] was compared to those who remained asymptomatic after 3 years [N = 30], there were significant differences [P < .05] in total scores on the Scale for the Assessment of Negative Symptoms, the Clinical Global Impressions scale, and the Disability Assessment Schedule.Conclusions: Antipsychotic treatment discontinuation in individuals who had accomplished a functional recovery after a single psychotic episode was associated with a high risk of symptom recurrence. Relapsed individuals had a greater severity of symptoms and lower functional status after 3 years.
Trial Registration: ClinicalTrials.gov identifier: NCT02220504Author contributors: Drs Mayoral-van Son, Parrilla-Escobar, and Moreno collected clinical data. Dr Mayoral-van Son interpreted the results and drafted the manuscript. Mr Ortiz-Garcia de la Foz performed the statistical analyses and drafted the manuscript. Dr Crespo-Facorro interpreted the results and reviewed the manuscript. Drs Valdizan and Martinez-Garcia helped in the interpretation of clinical data and reviewed the manuscript. The corresponding author had access to all study data. All authors approved the final version of the manuscript.Potential conflicts of interest: Dr Crespo-Facorro has received honoraria for his participation as a speaker at educational events from Otsuka, Lundbeck, and Johnson & Johnson. Drs Mayoral-van Son, Valdizan, Parrilla, Moreno, and Martinez-Garcia and Mr Ortiz-Garcia de la Foz report no additional financial or other relationship relevant to the subject of this article.Funding/support: The present study was conducted at the Hospital Marqués de Valdecilla, University of Cantabria, Santander, Spain, under the following grant support: SENY Fundación Research Grant CI 2005-0308007 and Fundación Marqués de Valdecilla API07/011.
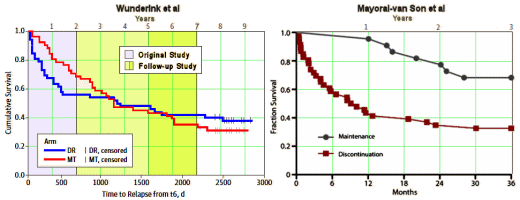
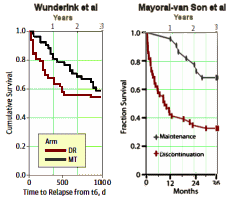 … and then again in a scaled-and-colored-to-match version to show the difference in parallel. For review, Wunderink et al’s initial report after two years found what has traditionally been reported – that maintenance medication had a significant positive effect on relapse rates. However, in a later follow-up, they found that the relapse rates equalized [from about three years on], and on functional testing, the patients who were not on a fixed maintenance schedule actually came out better by a respectable margin at about seven years. This new study from Spain did not replicate Wunderink’s findings:
… and then again in a scaled-and-colored-to-match version to show the difference in parallel. For review, Wunderink et al’s initial report after two years found what has traditionally been reported – that maintenance medication had a significant positive effect on relapse rates. However, in a later follow-up, they found that the relapse rates equalized [from about three years on], and on functional testing, the patients who were not on a fixed maintenance schedule actually came out better by a respectable margin at about seven years. This new study from Spain did not replicate Wunderink’s findings:A systematic review of studies exploring the recurrence of psychotic symptoms with antipsychotic discontinuation in first-episode nonaffective psychosis reported a weighted mean recurrence rate of 77% during the first year and more than 90% at 2 years.33 In contrast, the 1-year risk of recurrence within the medication continuation group was estimated at 3%. These estimates differed considerably from the 1-year rates of relapse in FEP patients of 61% in the discontinuation group and 26% in the continuation medication group reported by Leucht and colleagues.1 Methodological discrepancies in the study design; inclusion criteria; and duration of follow-up, diagnosis, and hospitalization rates make comparability between studies problematic. Wunderink and colleagues, exploring remitted psychotic patients, observed that relapse rates were 2 times higher in individuals who gradually tapered or discontinued medication, and that only 20% of patients could be successfully discontinued. Strikingly, those patients with an earlier reduction or discontinuation treatment strategy appear to have long-term functional gains compared with individuals who maintained treatment. Stopping antipsychotic medication has been repeatedly demonstrated as the biggest predictor of relapse in schizophrenia. It is of note that, despite most relapsed patients’ responding promptly to resuming antipsychotics, our data herein indicate that patients who suffered a relapse had a decreased functional status and a greater severity of symptomatology at 3 years compared with those patients who did not relapse.
But the general tone of the discussions about maintenance medications in psychotic illness has been so contentious for so long that it would be naive to think that any one study will bring things to any resolution. The dialog has been too divisive to expect anything to be that simple. The issues of maintenance antipsychotics, involuntary hospitalization, forced drugging, commercialization, conflicts of interest, the medical model, guild vs guild, the recovery and survivor movements, etc. have gotten all glommed up together and made productive dialog difficult at best and, at times, impossible [see persistence…]. But the resolution between the implications of those two graphs, these two studies, isn’t a polemic difference – it’s science, a science that needs to be worked out scientifically.
As I’ve said before, I started out believing that aiming to be medication-free was the best of ideas following a psychotic episode, but my experience was different. I had believed that working together, the patient and I could identify triggers and incipient symptoms, and prevent or outrun relapses. But that was the exception rather than the rule in my [again] limited experience. In this study, they found what I saw – that the onset of relapse occurred with little or no prodrome, and the relapse itself was more than just disruptive – it was destructive. My perspective is [again] anecdotal, but theirs comes from a more scientific observational frame.
Antidepressant Use During Pregnancy and the Risk of Autism Spectrum Disorder in Childrenby Takoua Boukhris, Odile Sheehy, Laurent Mottron, and Anick BérardJAMA Pediatrics. 2015/. Published online December 14, 2015.
IMPORTANCE The association between the use of antidepressants during gestation and the risk of autism spectrum disorder [ASD] in children is still controversial. The etiology of ASD remains unclear, although studies have implicated genetic predispositions, environmental risk factors, and maternal depression.OBJECTIVE To examine the risk of ASD in children associated with antidepressant use during pregnancy according to trimester of exposure and taking into account maternal depression.DESIGN, SETTING, AND PARTICIPANTS We conducted a register-based study of an ongoing population-based cohort, the Québec Pregnancy/Children Cohort, which includes data on all pregnancies and children in Québec from January 1, 1998, to December 31, 2009. A total of 145 456 singleton full-term infants born alive and whose mothers were covered by the Régie de l’assurance maladie du Québec drug plan for at least 12 months before and during pregnancy were included. Data analysis was conducted from October 1, 2014, to June 30, 2015.EXPOSURES Antidepressant exposure during pregnancy was defined according to trimester and specific antidepressant classes.MAIN OUTCOMES AND MEASURES Children with ASD were defined as those with at least 1 diagnosis of ASD between date of birth and last date of follow-up. Cox proportional hazards regression models were used to estimate crude and adjusted hazard ratios with 95%CIs.RESULTS During 904 035.50 person-years of follow-up, 1054 children [0.7%] were diagnosed with ASD; boys with ASD outnumbered girls by a ratio of about 4:1. The mean [SD] age of children at the end of follow-up was 6.24 [3.19] years. Adjusting for potential confounders, use of antidepressants during the second and/or third trimester was associated with the risk of ASD [31 exposed infants; adjusted hazard ratio, 1.87; 95%CI, 1.15-3.04]. Use of selective serotonin reuptake inhibitors during the second and/or third trimester was significantly associated with an increased risk of ASD [22 exposed infants; adjusted hazard ratio, 2.17; 95%CI, 1.20-3.93]. The risk was persistent even after taking into account maternal history of depression [29 exposed infants; adjusted hazard ratio, 1.75; 95%CI, 1.03-2.97].CONCLUSIONS AND RELEVANCE Use of antidepressants, specifically selective serotonin reuptake inhibitors, during the second and/or third trimester increases the risk of ASD in children, even after considering maternal depression. Further research is needed to specifically assess the risk of ASD associated with antidepressant types and dosages during pregnancy.
[Note: I added the bottom line – the % of their pregnancy cohort on ADs]
We worry a lot about drugs being promoted for commercial reasons. I even implied that earlier talking about Drs. Nemeroff and Stowe. But there’s another force that’s more benevolent to consider – therapeutic zeal. The OBs that I knew back then weren’t in that entrepreneurial camp at all. They were solid citizens who were genuinely worried about their patients’ plight. And I expect that the doctors and pharmacists in Germany [where Thalidomide became OTC] were using it benevolently. Hyperemesis Gravidarum can be an absolutely miserable and sometimes dangerous symptom of pregnancy, and I would bet Thalidomide was hailed as a real breakthrough [until its dreadful outcomes became apparent].
So let’s first look at an example using a continuous variable comparing two different groups. The information we started with is in the left column and the values we calculated to derive the Effect Size [Cohen’s d] are in the right column [see in the land of sometimes[1]…]:
|
Group Statistics
|
Effect Size Calculations
|
|||||
| subjects | n1, n2 | Cohen’s d | d = (μ1 – μ2) ÷ σ | |||
| means | μ1, μ2 | pooled std dev | ||||
| std devs | σ1, σ2 | |||||
 Remember that Cohen’s d is also known as the Standardized Mean Difference. So what we have in the right hand column is actually the distribution of the Effect Size. All we have to do now is realize that what we’ve been calling the Pooled Standard Deviation is actually the standard deviation of Cohen’s d, and that we can use it to calculate the 95% Confidence Limits using these simple formulas [either take that last part on faith or read it over several times until it makes sense]:
Remember that Cohen’s d is also known as the Standardized Mean Difference. So what we have in the right hand column is actually the distribution of the Effect Size. All we have to do now is realize that what we’ve been calling the Pooled Standard Deviation is actually the standard deviation of Cohen’s d, and that we can use it to calculate the 95% Confidence Limits using these simple formulas [either take that last part on faith or read it over several times until it makes sense]:
So now, instead of showing Cohen’s d as a frequency distribution like the upper right, we can display it more simply:
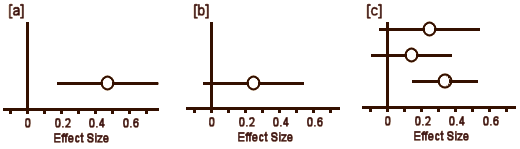
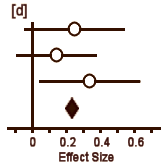 In [a], the circle represents Cohen’s d and the lines define the 95% Confidence Interval. So with a glance, you can see the Effect Size, the "spread," and whether it’s significant [the Confidence Interval line doesn’t touch or cross zero – the null hypothesis]. The example in [b] is not significant. But what I consider the brilliant part is in [c]. If you have three different studies of a drug versus placebo, you can display them together and again, in a glance, see what’s up with all of them – the essence of meta-analysis. And there’s even more. By weighting these studies eg based on sample sizes, one can display a summary Effect Size that represents the whole collection of studies [d]. In the case of using sample sizes:
In [a], the circle represents Cohen’s d and the lines define the 95% Confidence Interval. So with a glance, you can see the Effect Size, the "spread," and whether it’s significant [the Confidence Interval line doesn’t touch or cross zero – the null hypothesis]. The example in [b] is not significant. But what I consider the brilliant part is in [c]. If you have three different studies of a drug versus placebo, you can display them together and again, in a glance, see what’s up with all of them – the essence of meta-analysis. And there’s even more. By weighting these studies eg based on sample sizes, one can display a summary Effect Size that represents the whole collection of studies [d]. In the case of using sample sizes:
d = (n1 × d1 + n2 × d2 + n3 × d3) ÷ (n1 + n2 + n3)
 We’ve gotten so used to these forest plots that it’s easy to forget how much they’ve helped us see the big picture in one simple graphic. At least for me, this is a testimony to the adage, "a picture’s worth a thousand words." There’s something of a monotony in these clinical trials, so the information needed for this kind of comparison is almost always available. And, by the way, look at the Cochrane Collaboration logo.
We’ve gotten so used to these forest plots that it’s easy to forget how much they’ve helped us see the big picture in one simple graphic. At least for me, this is a testimony to the adage, "a picture’s worth a thousand words." There’s something of a monotony in these clinical trials, so the information needed for this kind of comparison is almost always available. And, by the way, look at the Cochrane Collaboration logo.
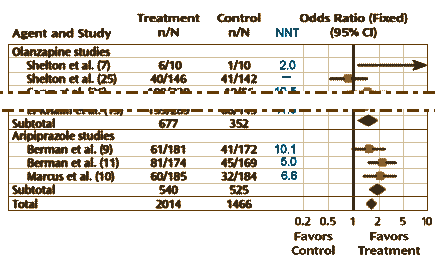
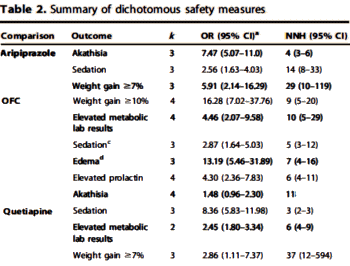
Well, what about the categorical variables? One can almost say "ditto." They use the Effect Size as well, in this case the Odds Ratio. The calculation of the 95% Confidence Intervals is less intuitive, so most mortals like me would be best advised to use one of the Internet calculators [here, here, and here]. The vertical line representing the null hypothesis is at 1 instead of 0. And there’s something else different: the 95% Confidence Interval lines are asymmetric, so authors usually use a logarithmic scale to display the values symmetrically. Look at the Odds Ratio scale on the meta-analysis of Atypical Antipsychotic augmentation I reported on earlier [creative funding III & some other things…]: