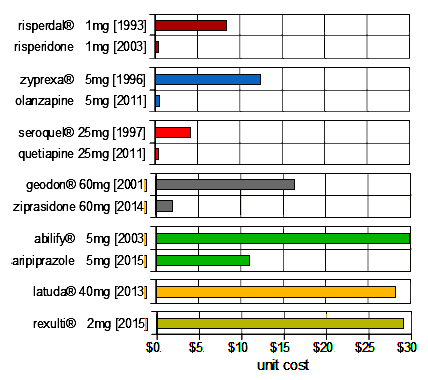
Pfizer’s Geodon® [Ziprasidone] was the fifth Atypical Anipsychotic approved [2001]. The
Orange Book lists its patent exclusivity through 2019, though it appears that
generics have been approved since 2012 that are now available [see
current costs] [
I’ve given up trying parse patent/exclusivity information, so that’s all I know]. Independent of that confusion, there’s a Clinical Trial in the December
American Journal of Psychiatry that deserves some attention –
Geodon® augmentation of
Lexapro® in
Treatment Resistant Depression. Here’s the abstract:
by Papakostas GI, Fava M, Baer L, Swee MB, Jaeger A, Bobo WV, and Shelton RC.
American Journal of Psychiatry. 2015 172[12]:1251-1258.
OBJECTIVE: The authors sought to test the efficacy of adjunctive ziprasidone in adults with nonpsychotic unipolar major depression experiencing persistent symptoms after 8 weeks of open-label treatment with escitalopram.
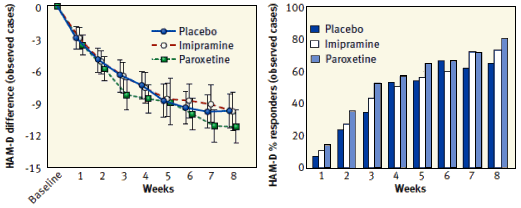
METHOD: This was an 8-week, randomized, double-blind, parallel-group, placebo-controlled trial conducted at three academic medical centers. Participants were 139 outpatients with persistent symptoms of major depression after an 8-week open-label trial of escitalopram [phase 1], randomly assigned in a 1:1 ratio to receive adjunctive ziprasidone [escitalopram plus ziprasidone, N=71] or adjunctive placebo [escitalopram plus placebo, N=68], with 8 weekly follow-up assessments. The primary outcome measure was clinical response, defined as a reduction of at least 50% in score on the 17-item Hamilton Depression Rating Scale [HAM-D]. The Hamilton Anxiety Rating scale [HAM-A] and Visual Analog Scale for Pain were defined a priori as key secondary outcome measures.
RESULTS: Rates of clinical response [35.2% compared with 20.5%] and mean improvement in HAM-D total scores [-6.4 [SD=6.4] compared with -3.3 [SD=6.2]] were significantly greater for the escitalopram plus ziprasidone group. Several secondary measures of antidepressant efficacy also favored adjunctive ziprasidone. The escitalopram plus ziprasidone group also showed significantly greater improvement on HAM-A score but not on Visual Analog Scale for Pain score. Ten [14%] patients in the escitalopram plus ziprasidone group discontinued treatment because of intolerance, compared with none in the escitalopram plus placebo group.
CONCLUSIONS: Ziprasidone as an adjunct to escitalopram demonstrated antidepressant efficacy in adult patients with major depressive disorder experiencing persistent symptoms after 8 weeks of open-label treatment with escitalopram.
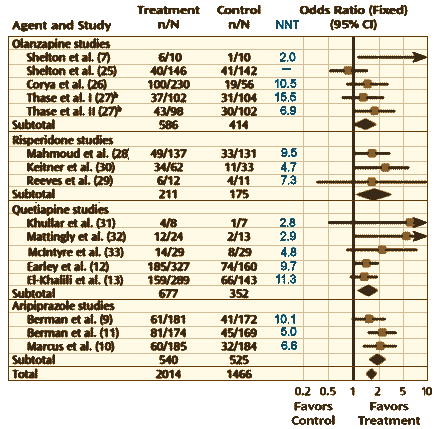
This article is typical of the indication creep that followed the initial approvals of the Atypical Antipsychotics as they flowed from the pipeline. First Schizophrenia for initial approval, then Mania, then a shot at monotherapy for Major Depressive Disorder or augmentation in cases that didn’t respond to SSRIs [AKA Treatment Resistant Depression] – accompanied by exper·o·mmercial articles designed to generate reprints for detail men to hand out on their visits. What’s different in this case is the funding:
Supported by the NIMH grant
R01MH081235, Pfizer [which supplied blinded ziprasidone and placebo pills], and Forest Laboratories [which supplied escitalopram].
DESCRIPTION [provided by applicant]: Identifying novel treatments for resistant depression [TRD] is urgently needed to help improve the standard of care. To date, several preliminary studies have examined the use of atypical antipsychotic agents as adjuncts to standard antidepressants for TRD. However, the efficacy of this popular off-label treatment strategy has yet to be firmly established, while very little is known regarding the long-term effects [in terms of efficacy, tolerability and safety] of this treatment strategy. The atypical antipsychotic agent ziprasidone, in particular, may offer a unique opportunity to study as an adjunct for TRD for two principal reasons: I] its unique receptor-affinity profile, and, II] its favorable side-effect profile compared to the other agents in the class. Unfortunately, however, double-blind, placebo controlled trials of ziprasidone augmentation for TRD have not been conducted to date. If safe and effective as an antidepressant adjunct, ziprasidone would represent an attractive option for many of these patients who have had unsatisfactory initial response to standard treatment. If not found to be either safe or effective, the results of this proposed trial would also be highly informative given the significant proportion of TRD patients who, despite
the relative paucity of data from independently-funded studies of rigorous design, are prescribed atypical antipsychotic agents off-label"…
The
NIMH Grant was awarded to
Richard Shelton [then at Vanderbilt] and ultimately came to
$1,656,479 over the 5 years [2008-2012]. The study was carried out in conjunction with Mass General’s trial network, Vanderbilt, and the University of Alabama [Shelton apparently moved from Vanderbilt to the University of Alabama]. Other than the fact that it was financed by the NIMH, it was not unlike all the other
indication creep exper·o·mmercials of the day – including a Conflict of Interest declaration [appended below] that should make anyone blush [note the presence of
Pfizer in 4/4 MD Authors].
As for the study itself, I have to comment that finding the information wasn’t easy. In the clinicaltrials.gov write-up [NCT00633399], the primary outcome variable was a fall in the HAM-D score of 50% during the 8 weeks on Geodon. The secondary outcome variables were a HAM-D score < 8 at Week 8, and Comparing Scores on HAM-D Baseline Visit to Phase 2 Final Visit at Week 8.  They reported the primary outcome variable significant at p = 0.04 and the p values for the secondary outcomes respectively as p = 0.32 and p = 0.04. Obviously without the data, these couldn’t be checked using fancy tests and software, but using a simple 2×2 test, I couldn’t confirm the primary p-value.
They reported the primary outcome variable significant at p = 0.04 and the p values for the secondary outcomes respectively as p = 0.32 and p = 0.04. Obviously without the data, these couldn’t be checked using fancy tests and software, but using a simple 2×2 test, I couldn’t confirm the primary p-value.
In their article, they mention other "secondary" outcome variables using the QIDS-SR and the HAM-A all of which are positive. Another bit of questionable reporting had to do with weight gain. It’s not mentioned in the abstract and in the body of the paper, it says:
There were no significant between-group differences in reported rates of sexual dysfunction or weight gain.
But then at the very [very] end of the paper, there was this:
Addendum: Additional analysis found a trend significance in QTc by 8.8 msec in the ziprasidone-tested group and a significant increase in weight gain of 3.5 kg in the ziprasidone-treated group versus 1.0 kg on placebo. Further information on these and other laboratory parameters is available from Dr. Papakostas [
gpapakostas@partners.org].
I almost passed over this article, but a quick glance at the funding and COI declarations at the end gave me pause. "
How in the world did they get the NIMH to fund this study?" was enough of a question to pique my interest. The argument [circa 2008] that "
the relative paucity of data from independently-funded studies of rigorous design" justified NIMH funding was certainly unexpected [and after nosing around, unjustified]. There’s nothing rigorous about the COI statement, or the analyses, or the add-on post-hoc variables, or weight gain being an afterthought, or the minimalist
results report in clinicaltrials.gov. I’m not sure that anyone much cares about the topic itself. It’s in the genre of those sequencing, combining, augmenting, personalizing studies trying to eke more out of the SSRIs than they apparently have – and as such is anachronistic. But I for one care that this study is in the
American Journal of Psychiatry in December of 2015. And I sure care that the
NIMH footed the bill. There’s an accompanying editorial [
Adjunctive Ziprasidone in Major Depression and the Current Status of Adjunctive Atypical Antipsychotics] as well [first page available
here].
I’m going to stop here for the moment, but I’m not done. There’s another article I would consider an indication creep exper·o·mmercial in the same issue of the AJP that’s NIMH funded. So after a breather, I’ll briefly mention the other one [also with its accompanying editorial] and comment on the two simultaneously in the next post.
Conflict of Interest Statements
Dr. Papakostas has received research support from or served as a consultant or speaker for Abbott, AstraZeneca, Avanir, Brainsway, Bristol-Myers Squibb, Cephalon, Dey Pharma, Eli Lilly, Forest, Genentech, GlaxoSmithKline, Evotec AG, Lundbeck, Inflabloc, Janssen Global Services, Jazz Pharmaceuticals, Johnson & Johnson, NIMH, Novartis, One Carbon Therapeutics, Otsuka, Pamlab, Pfizer, Pierre Fabre, Ridge Diagnostics [formerly known as Precision Human Biolaboratories], Shire, Sunovion, Takeda, Theracos, Titan Pharmaceuticals, and Wyeth.
Dr. Fava has received research support from or served on advisory boards or as a consultant for Abbott, Affectis Pharmaceuticals, Alkermes, Amarin Pharma, American Cyanamid, Aspect Medical Systems, AstraZeneca, Auspex, Avanir, AXSOME Therapeutics, Bayer AG, Best Practice Project Management, BioMarin Pharmaceuticals, BioResearch, Biovail Corporation, BrainCells, Bristol-Myers Squibb, CeNeRx BioPharma, Cephalon, Cerecor, Clintara, CNS Response, Compellis Pharmaceuticals, Covance, Covidien, Cypress Pharmaceutical, Dainippon Sumitomo, DiagnoSearch Life Sciences, Dov Pharmaceuticals, Edgemont Pharmaceuticals, Eisai, Eli Lilly, EnVivo Pharmaceuticals, ePharmaSolutions, EPIX Pharmaceuticals, Euthymics Bioscience, Fabre-Kramer Pharmaceuticals, Forest Pharmaceuticals, Forum Pharmaceuticals, Ganeden Biotech, GenOmind, GlaxoSmithKline, Grunenthal GmbH, Harvard Clinical Research Institute, Hoffman-LaRoche, Icon Clinical Research, i3 Innovus/Ingenix, Janssen, Jazz Pharmaceuticals, Jed Foundation, Johnson & Johnson, Knoll Pharmaceuticals, Labopharm, Lichtwer Pharma GmbH, Lorex, Lundbeck, MedAvante, Merck, MSI Methylation Sciences, NARSAD, National Center for Complementary and Alternative Medicine, Naurex, Nestlé Health Sciences, Neuralstem, Neuronetics, NextWave Pharmaceuticals, NIDA, NIMH, Novartis AG, Nutrition 21, Orexigen Therapeutics, Organon, Otsuka, Pamlab, Pfizer, Pharmacia-Upjohn, Pharmaceutical Research Associates, PharmaStar, Pharmavite, PharmoRx Therapeutics, Photothera, Precision Human Biolaboratory, Prexa Pharmaceuticals, PPD, Puretech Ventures, PsychoGenics, Psylin Neurosciences, Reckitt Benckiser, Rexahn Pharmaceuticals, Ridge Diagnostics, Roche Pharmaceuticals, RCT Logic [formerly Clinical Trials Solutions], Sanofi-Aventis, Schering-Plough, Sepracor, Servier Laboratories, Shire, Solvay, Somaxon, Somerset, Stanley Medical Research Institute, Sunovion, Supernus, Synthelabo, Takeda, Tal Medical, Tetragenex, TransForm Pharmaceuticals, Transcept Pharmaceuticals, Vanda Pharmaceuticals, and Wyeth-Ayerst; he has served as a speaker or author for Adamed, Advanced Meeting Partners, American Psychiatric Association, American Society of Clinical Psychopharmacology, AstraZeneca, Belvoir Media Group, Boehringer Ingelheim GmbH, Bristol-Myers Squibb, Cephalon, CME Institute/Physicians Postgraduate Press, Eli Lilly, Forest Pharmaceuticals, GlaxoSmithKline, Imedex, MGH Psychiatry Academy/Primedia, MGH Psychiatry Academy/Reed Elsevier, Novartis, Organon, Pfizer, PharmaStar, United BioSource, and Wyeth-Ayerst Laboratories; he has equity holdings in Compellis and PsyBrain; he receives royalty, patent, or other income for patents for sequential parallel comparison design, licensed by MGH to Pharmaceutical Product Development, and has a patent application for a combination of ketamine plus scopolamine in major depressive disorder, licensed by MGH to Biohaven; he is a copyright holder for the MGH Cognitive and Physical Functioning Questionnaire, Sexual Functioning Inventory, Antidepressant Treatment Response Questionnaire, Discontinuation-Emergent Signs and Symptoms, Symptoms of Depression Questionnaire, and SAFER; and he receives royalties from Lippincott Williams & Wilkins, Wolkers Kluwer, and World Scientific Publishing.
Dr. Bobo has received research support from Cephalon, the Mayo Foundation, NARSAD, and NIMH and has served on speakers bureaus for Janssen and Pfizer.
Dr. Shelton has received research support from or served as a consultant for Alkermes, Assurex Health, Avanir Pharmaceuticals, Bristol-Myers Squibb, Cerecor, Clintara, Cyberonics, Elan, Forest Pharmaceuticals, Janssen, Medtronic, MSI Methylation Sciences, Naurex, Nestlé Health Sciences–Pamlab, Novartis, Otsuka, Pfizer, Ridge Diagnostics, Shire, and Takeda.
The other authors report no financial relationships with commercial interests.
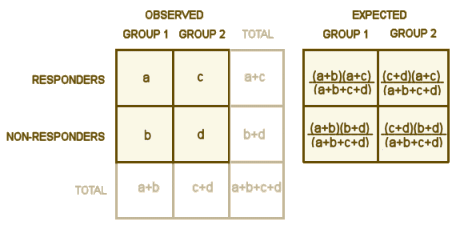
 … and add the four values to get the X2 test statistic If this were 1971, you’d take the test statistic and the degrees of freedom [rows-1] × [columns-1] and look up the p value in a book of statistical tables. But it’s not 1971, it’s 2015. So you’ll forget all the calculating and use an Internet calculator like vasserstats by simply filling in a, b, c, and d, and like magic the p values will just appear. There’s a bigger calculator if you have more than two groups. There are some subtleties [Pearson’s, Fisher’s, Yates’s] that I can’t keep quite straight myself from time to time, but they are easy and well explained on the Wikipedia and vassarstats pages. Thanks to the Internet, the p value is just seconds away.
… and add the four values to get the X2 test statistic If this were 1971, you’d take the test statistic and the degrees of freedom [rows-1] × [columns-1] and look up the p value in a book of statistical tables. But it’s not 1971, it’s 2015. So you’ll forget all the calculating and use an Internet calculator like vasserstats by simply filling in a, b, c, and d, and like magic the p values will just appear. There’s a bigger calculator if you have more than two groups. There are some subtleties [Pearson’s, Fisher’s, Yates’s] that I can’t keep quite straight myself from time to time, but they are easy and well explained on the Wikipedia and vassarstats pages. Thanks to the Internet, the p value is just seconds away.

 – but they can take a depression medicine. If it doesn’t have the desired effect, they’re understandably disappointed and want to try something else. They know that it’s out there as seen on television, but they think they just haven’t found it yet. So like in the above example, one is well advised to develop a similar pre-emptive line.
– but they can take a depression medicine. If it doesn’t have the desired effect, they’re understandably disappointed and want to try something else. They know that it’s out there as seen on television, but they think they just haven’t found it yet. So like in the above example, one is well advised to develop a similar pre-emptive line.

 I so rarely disagree with anything Ed Silverman of Pharmalot has to say, I relish the opportunity to disagree with him for a change. When I read that the AMA had voted to call for a ban on Direct-to-Consumer [DTC] ads on TV [
I so rarely disagree with anything Ed Silverman of Pharmalot has to say, I relish the opportunity to disagree with him for a change. When I read that the AMA had voted to call for a ban on Direct-to-Consumer [DTC] ads on TV [


 While it is unlikely that Martin Shkreli will ever be canonized like
While it is unlikely that Martin Shkreli will ever be canonized like 
