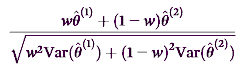I suppose that with the drought in the PHARMA CNS pipeline, it should have been predictable that thoughts would turn towards the known feel-good drugs [drugs in the street-sense as opposed to medications]. In October, we heard about Special K, a club drug, looking to be reborn as a antidepressant …
by D. Jeffrey Newport, Linda L. Carpenter, William M. McDonald, James B. Potash, Mauricio Tohen, and Charles B. Nemeroff, The APA Council of Research Task Force on Novel Biomarkers and Treatments
American Journal of Psychiatry. 2015 172:950–966.
… Other NMDA antagonists failed to consistently demonstrate efficacy; however, two partial agonists at the NMDA coagonist site, D-cycloserine and rapastinel, significantly reduced depressive symptoms without psychotomimetic or dissociative effects.
Conclusions: The antidepressant efficacy of ketamine, and perhaps D-cycloserine and rapastinel, holds promise for future glutamate-modulating strategies; however, the ineffectiveness of other NMDA antagonists suggests that any forthcoming advances will depend on improving our understanding of ketamine’s mechanism of action. The fleeting nature of ketamine’s therapeutic benefit, coupled with its potential for abuse and neurotoxicity, suggest that its use in the clinical setting warrants caution.
… with emphasis on a couple of derivative drugs that weren’t so "
clubby" [that also have a significant commercial potential and obvious author Conflicts of Interest – see
a touch of paralysis… and
infomercials…]. Now, there’s a lot of excitement around exploiting an old standby – the opiate receptors. Interestingly, it comes at a time of an epidemic of opiate abuse – traffic coming
straight from the poppies and from the pharmaceutical
pain pills. With my blog about Dr. Nemeroff et al’s Ketamine article, I spread the COI declarations throughout the text. Those connections are here too and I’m going to stick them in at the end. But to summarize them, the clinical trial from this month’s AJP is industry financed, has four academic AKA known KOL authors all with connections with the drug manufacturer, and seven authors who are actually full time employees.
by Maurizio Fava, M.D., Asli Memisoglu, Sc.D., Michael E. Thase, M.D., J. Alexander Bodkin, M.D., Madhukar H. Trivedi, M.D., Marc de Somer, M.D., M.P.H., Yangchun Du, Ph.D., Richard Leigh-Pemberton, M.D., Lauren DiPetrillo, Ph.D., Bernard Silverman, M.D., Elliot Ehrich, M.D.
American Journal of Psychiatry. Published online: February 12, 2016
Objective: Major depressive disorder has been associated with dysregulation of the endogenous opioid system. The authors sought to determine whether opioid modulation achieved through administration of ALKS 5461, a combination of an m- and k-opioid partial agonist, buprenorphine, and an opioid antagonist, samidorphan, would exhibit antidepressant activity in patients with major depression.
Method: A multicenter, randomized, double-blind, placebo- controlled, two-stage sequenti al parallel comparison design study was conducted in adults with major depression who had an inadequate response to one or two courses of antidepressant treatment. Participants were randomly assigned to receive adjunctive treatment with 2mg/2mg of buprenorphine/samidorphan [the 2/2 dosage group], 8mg/8mg of buprenorphine/samidorphan [the 8/8 dosage group], or placebo. Antidepressant effect was measured based on change from baseline to the end of 4 weeks of treatment on the 17-item Hamilton Depression Rating Scale [HAM-D], the Montgomery-Åsberg Depression Rating Scale [MADRS], and the Clinical Global Impressions severity scale [CGI-S].
Results: Compared with the placebo group, there were significantly greater improvements in the 2/2 dosage group across the three depression outcome measures [HAM-D: -2.8, 95% CI= -5.1, -0.6; MADRS: -4.9, 95%CI= -8.2, -1.6; CGI-S: -0.5, 95% CI= -0.9, -0.1]. There was also evidence of improvement in the 8/8 dosage group, although it did not achieve statistical significance. Overall, the buprenorphine/samidorphan combinations were well tolerated, and there was no evidence of opioid withdrawal on treatment discontinuation.
Conclusions: The buprenorphine/samidorphan combination is a novel and promising candidate for treatment of major depressive disorder in patients who have an inadequate response to standard antidepressants.
The study above, reported in the American Journal of Psychiatry today was Received: July 15, 2015 and Accepted: November 30, 2015. It was one of those earlier studies that generated all that excitement on Wall Street. It was a "Two-Stage Sequential Parallel Comparison Design Study" – it’s something of a crossover design but too complex to explain without reproducing the whole article. The same for the analysis. Speaking of complex, they had a fancy way of evaluating their crossover data that included this $50 formula:
[I sure didn’t get it]. In the end, they reported a significant difference for the lower but not the higher dose of their drug combo [HAM-D p=0.02, MADRS p=0.005, CGI p=0.03]. Of note, there were no significant differences in the patient self rating scales [Inventory of Depressive Symptomatology Self-Report, the Sheehan Disability Scale, or the SF-12].
It’s hard for me to imagine that they’ll ever succeed in engineering an opiate that has some therapeutic effect without the obvious problem of abuse and addiction – the kind that practitioners already deal with every day. I literally surrendered the narcotic part of my narcotic license in order to be able to say "I can’t prescribe pain pills." I was just so weary from being hit on for narcotics. And frankly, it feels a lot like the deepest theory herein is to over-ride depressive affect with the euphoric effect of opium. That experiment has already gone on for centuries without coming to any good end.

AUTHOR AND ARTICLE INFORMATION
From the Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston; Alkermes, Inc., Waltham, Mass.; the Department of Psychiatry, University of Pennsylvania School of Medicine, Philadelphia; McLean Hospital, Belmont, Mass.; and the Department of Psychiatry, University of Texas Southwestern Medical Center, Dallas. Address correspondence to Dr. Fava [mfava@partners.org].
Presented at the 53rd annual meeting of the New Clinical Drug Evaluation Unit, Hollywood, Fla., May 28 – 31, 2013.
Supported by Alkermes, Inc., Waltham, Mass. The authors thank Mark S. Todtenkopf, Ph.D., for assistance in the preparation of the manuscript; the ALKS 5461 investigator study group; and Richard S. Perry, Pharm.D., for editorial assistance in the preparation of the manuscript, which was supported by Alkermes, Inc.
Dr. Fava has received research support from and/or served as an adviser or consultant to Acadia, Alkermes, AstraZeneca, Avanir, AXSOME Ther- apeutics, Biogen, Bristol-Myers Squibb, Cerecor, Dinippon Sumitomo Pharma, Eli Lilly, EnVivo, Euthymics Bioscience, Forest Pharmaceuticals, FORUM Pharmaceuticals, GenOmind, GlaxoSmithKline, Intracellular, Janssen R&D, Johnson & Johnson Pharmaceutical Research and De- velopment, Lundbeck, Merck, Methylation Sciences, MSI Methylation Sciences, the National Center for Complementary and Alternative Med- icine, the National Coordinating Center for Integrative Medicine, NIDA, NIMH, Naurex, Nestle Health Sciences, Neuralstem, Novartis AG, Nutrition 21, Osmotica, Otsuka Pharmaceuticals, PamLab, Pfizer, PharmoRx Therapeutics, Photothera, PPD, Puretech Ventures, PsychoGenics, RCT Logic [formerly Clinical Trials Solutions], Reckitt Benckiser, Ridge Diagnostics, Roche Pharmaceuticals, Sanofi-Aventis, Servier Laboratories, the Stanley Medical Research Institute, Sunovion, Taisho, Takeda, Tal Medical, and VistaGen; he has had speaking or publishing roles for the American Society of Clinical Psychopharmacology, Belvoir Media Group, CME Institute/Physicians Postgraduate Press, and MGH Psychiatry Academy; he has equity holdings in Compellis and PsyBrain; he is named on patents for sequential parallel comparison design, licensed by MGH to Pharma- ceutical Product Development and a patent application for a combination of ketamine plus scopolamine in major depressive disorder, licensed by MGH to Biohaven; and he is a copyright holder for the MGH Cognitive and Physical Functioning Questionnaire, the Sexual Functioning Inventory, the Antidepressant Treatment Response Questionnaire, Discontinuation-Emergent Signs and Symptoms, the Symptoms of Depression Questionnaire, and SAFER and has publications with Lippincott Williams & Wilkins, Wolkers Kluwer, and World Scientific Publishing.
Dr. Thase has served as an adviser or consultant to Alkermes, Allergan [includes Forest Laboratories and Naurex], Avanir, AstraZeneca, Bristol-Myers Squibb, Cerecor, Eli Lilly, Lundbeck, Johnson & Johnson [includes Ortho-McNeil and Janssen], MedAvante, Merck [includes Schering-Plough and Organon], Nestlé [includes Pamlab], Neuronetics, Novartis, Otsuka, Pfizer [includes Wyeth Ayerst], Roche, Sunovion, and Takeda; he has received grant support from Agency for Healthcare Research and Quality, Alkermes, Avenir, Eli Lilly, Forest, Johnson & Johnson, NIMH, Otsuka, Phar- maNeuroboost, and Roche; he has equity holdings in MedAvante and receives royalties from American Psychiatric Foundation, Guilford Publications, Herald House, and W.W. Norton; his spouse is employed by Peloton Advantage, which does business with Pfizer.
Dr. Bodkin has received payment for participation in advisory panel meetings related to the investigation of ALKS 5461 as a potential treatment for psychiatric disorders and has served as a consultant and received research support from Bristol-Myers Squibb, Otsuka, and CeNeRx, served as a consultant to MyTomorrows, has received research support from Shire, and has received grant support from NIH/NIMH.
Dr. Trivedi has served as a consultant to or received fees from Alkermes, AstraZeneca, Bristol-Myers Squibb, Cerecor, Eli Lilly, Forest, Johnson & Johnson, Lundbeck, Merck, Naurex, Neuronetics, Otsuka, Pamlab, Pfizer, Rexahn, Roche, and Shire and has served on a scientific advisory board for Rexahn; he has received research support from NIMH and NIDA.
Drs. Memisoglu, de Somer, Du, Leigh-Pemberton, DiPetrillo, Silverman, and Ehrich are or have been full-time employees of Alkermes, Inc., and have stock options.



 The Physicians Desk Reference [PDR] has grown since it was introduced in 1947, the year I started the first grade. It was still pretty thin when I started medical school in 1963, just a year after the Kefauver-Harris Drug Efficacy Act passed in the aftermath of the Thalidamide affair. The Act charged the FDA with certifying efficacy by at least two well conducted clinical trials in addition to its traditional safety evaluations.
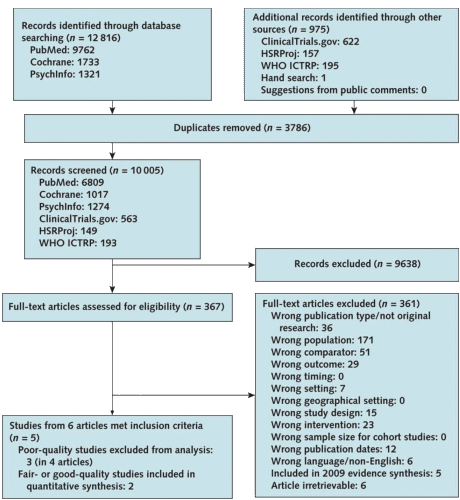
The Physicians Desk Reference [PDR] has grown since it was introduced in 1947, the year I started the first grade. It was still pretty thin when I started medical school in 1963, just a year after the Kefauver-Harris Drug Efficacy Act passed in the aftermath of the Thalidamide affair. The Act charged the FDA with certifying efficacy by at least two well conducted clinical trials in addition to its traditional safety evaluations.  Then in the 1980s, again in response to pressure, this time from the HIV victims, there was a movement to publicly register Clinical Trials. Those efforts culminated in the FDA Modernization act of 1997 that created clinicaltrials.gov – a public registry. It was originally conceived as placing parentheses around a trial [a registration database up front and reporting database when completed], but only the registration actually caught on. The post-completion reporting database cupboard is bare, and has been, even in situations where reporting is mandatory within one year, like in government funded trials. They just didn’t do it. My accounting only covers high points, but it gives the flavor of how things have gone – a chess game that doesn’t seem to end. And a PDR that just keeps on growing – filling with drugs some of whose efficacy and safety have been exaggerated…
Then in the 1980s, again in response to pressure, this time from the HIV victims, there was a movement to publicly register Clinical Trials. Those efforts culminated in the FDA Modernization act of 1997 that created clinicaltrials.gov – a public registry. It was originally conceived as placing parentheses around a trial [a registration database up front and reporting database when completed], but only the registration actually caught on. The post-completion reporting database cupboard is bare, and has been, even in situations where reporting is mandatory within one year, like in government funded trials. They just didn’t do it. My accounting only covers high points, but it gives the flavor of how things have gone – a chess game that doesn’t seem to end. And a PDR that just keeps on growing – filling with drugs some of whose efficacy and safety have been exaggerated… 
 So as the world of drug testing has become increasingly distant from academic influence and control, why are the academic authors still involved? My guess is that they bring the aura of an academic standard and a medical ethic to the trials, and, of course, serve as a ticket into the traditional academic journals. To my mind, this meta-analysis is just further evidence that that influence is simply a mirage. There are many other more effective ways to insure that product testing is on the up and up. The academic community would be more usefully engaged in vetting the data that these trials produce, disconnected from the manufacturers.
So as the world of drug testing has become increasingly distant from academic influence and control, why are the academic authors still involved? My guess is that they bring the aura of an academic standard and a medical ethic to the trials, and, of course, serve as a ticket into the traditional academic journals. To my mind, this meta-analysis is just further evidence that that influence is simply a mirage. There are many other more effective ways to insure that product testing is on the up and up. The academic community would be more usefully engaged in vetting the data that these trials produce, disconnected from the manufacturers.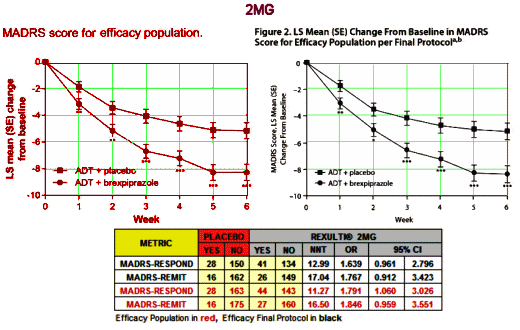
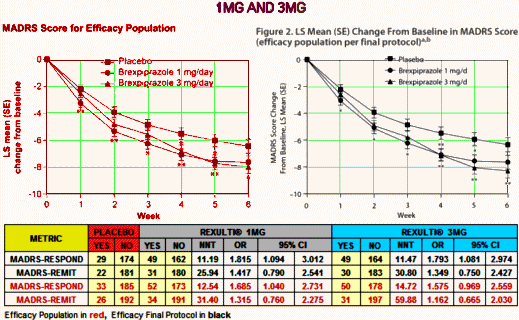
 I was writing about Harlan Krumholtz’s paper in the BMJ, and took a break to watch The Blacklist episode I missed [on the DVR]. About two-thirds through, I was fast-forwarding through the commercials but I was stopped in my tracks as a logo I recognized flashed by – Rexulti®. So I watched the ad and found myself screaming at the television set. In case you forgot about it, it’s the Abilify clone, the one that had two articles in succession in the JCP that were cut and paste copies, only one academic author [KOL Michael Thase], and was approved by the FDA as an adjunct for Treatment Resistant Depression, in spite of not making it without jury-rigging the results. For review:
I was writing about Harlan Krumholtz’s paper in the BMJ, and took a break to watch The Blacklist episode I missed [on the DVR]. About two-thirds through, I was fast-forwarding through the commercials but I was stopped in my tracks as a logo I recognized flashed by – Rexulti®. So I watched the ad and found myself screaming at the television set. In case you forgot about it, it’s the Abilify clone, the one that had two articles in succession in the JCP that were cut and paste copies, only one academic author [KOL Michael Thase], and was approved by the FDA as an adjunct for Treatment Resistant Depression, in spite of not making it without jury-rigging the results. For review:  I’ve read all the studies reviewed in 2009 and this current version. There’s not even a hint of a rational reason to recommend a preventive intervention in adolescent depression, and what these USPSTF papers conclude is as much bull-shit as some of the papers themselves. I’ve read a lot of the papers in the adult articles too. Same deal. So when I think back on my soldier days in the early 1970s, I wish I’d had my present mind and spoke it. Our Base Commander wasn’t thinking about his troops’ health. He was thinking about how they looked on inspections, or about being strong on the "regs," or about getting that star he wanted on his epaulets. And the reason he wasn’t going to get the star was widely known by all – his affinity for bourbon – which made his subsequent campaign even sillier.
I’ve read all the studies reviewed in 2009 and this current version. There’s not even a hint of a rational reason to recommend a preventive intervention in adolescent depression, and what these USPSTF papers conclude is as much bull-shit as some of the papers themselves. I’ve read a lot of the papers in the adult articles too. Same deal. So when I think back on my soldier days in the early 1970s, I wish I’d had my present mind and spoke it. Our Base Commander wasn’t thinking about his troops’ health. He was thinking about how they looked on inspections, or about being strong on the "regs," or about getting that star he wanted on his epaulets. And the reason he wasn’t going to get the star was widely known by all – his affinity for bourbon – which made his subsequent campaign even sillier.