![Gregor Mendel [1822-1884] Gregor Mendel [1822-1884]](http://1boringoldman.com/images/mendel-1.gif)
 Gregor Mendel was an Augustinian Monk in Moravia whose studies of plant inheritance brought such things into the realm of science. My sister’s blue eyes meant that both of our brown-eyed parents carried recessive blue-eye genes. The postman was out of the picture. Mendel’s schemes of inheritance were like that – very precise statistical predictions based on dominant genes. Things stayed pretty clean through Watson and Crick’s images of the double helix DNA structure, even Nirenberg’s nucleotide code sequences. But then things got out of hand – the Genome…
Gregor Mendel was an Augustinian Monk in Moravia whose studies of plant inheritance brought such things into the realm of science. My sister’s blue eyes meant that both of our brown-eyed parents carried recessive blue-eye genes. The postman was out of the picture. Mendel’s schemes of inheritance were like that – very precise statistical predictions based on dominant genes. Things stayed pretty clean through Watson and Crick’s images of the double helix DNA structure, even Nirenberg’s nucleotide code sequences. But then things got out of hand – the Genome…
That’s something I said this time last year in a post criticizing Dr. Insel’s NIMH and his funding priorities. It was after the Psychiatric Genomics Consortium report: Biological insights from 108 schizophrenia-associated genetic loci. I’m not competent to judge these "big data" studies coming from genomic research, but I am competent to complain that the NIMH isn’t looking into the plight of our psychotic patients living in prison – and that’s what I was addressing in those posts. I was looking for balance from the NIMH [in vain, I might add].
This week, psychologist Jay Joseph who writes on Mad in America, adds [Are DSM Psychiatric Disorders “Heritable”?] to his series of posts [the list] and books [The Gene Illusion: Genetic Research in Psychiatry and Psychology under the Microscope, The Missing Gene: Psychiatry, Heredity, and the Fruitless Search for Genes, The Trouble with Twin Studies: A Reassessment of Twin Research in the Social and Behavioral Sciences] challenging the psychiatric research and perspective on the genetics of mental illness. While looking over his posts, I came across one in particular that I’ve wanted to say something about for a while – DSM-5’s “Speculative” 2002 Diagnostic System Based On Expected Gene Findings.  He’s referring to a book [A Research Agenda for DSM-V] that I think has been too quickly forgotten. The book was published in 2002 edited by David Kupfer, Darrel Regier, and Michael First but had multiple contributers. Kupfer and Regier later became the co-chairmen of the DSM-5 Task Force. The premise of the book was that the previous DSMs had been based on descriptive criteria [atheoretical], and that it needed to be revised to include the biological bases for the various disorders. Jay’s post is pointing to a scheme in that book speculating about classifying mental disorders based on their genotypes:
He’s referring to a book [A Research Agenda for DSM-V] that I think has been too quickly forgotten. The book was published in 2002 edited by David Kupfer, Darrel Regier, and Michael First but had multiple contributers. Kupfer and Regier later became the co-chairmen of the DSM-5 Task Force. The premise of the book was that the previous DSMs had been based on descriptive criteria [atheoretical], and that it needed to be revised to include the biological bases for the various disorders. Jay’s post is pointing to a scheme in that book speculating about classifying mental disorders based on their genotypes:

At the time this was written, we didn’t know that any major mental condition was either biologic or genetic – though there was suggestive indirect evidence for some. We certainly had no clue about any genotypes. This book introduced a series of [extensive and expensive] symposiums funded by the APA and the NIMH to pursue the outlined agenda [see DSM-5 retrospective I…]. This enterprise – the book and the symposiums – was quite a reach beyond the DSM-III and DSM-IV. It aimed to move away from Spitzer’s reliability to the elusive dream of validity. And it made a broad assumption of biologic causality, not as a hypothesis, but as an as-yet-unconfirmed fact – at least that’s how it sounded to me.
I didn’t read that book until a few years after it was published and the symposiums had been completed, but all I could think about while reading it was why they ever thought they could make a leap of that magnitude between 2002 and 2010, or even come close. Why climb out on that limb? The only thing I could think of was that it was conceived around the time that the Human Genome Project was nearing completion, and they genuinely thought that locating the genotype of psychiatric syndromes would a snap. Further, looking at that Table 2-5, they were laying out a path of discovery and the discoveries to follow that was pretty specific – what kind of genes they were going to find and where they were going to be headed with this cascade of future findings – particularly in relationship to "targeted pharmacotherapy." As we now know, this enterprise was a definitive and very public flop. They had to admit defeat in March 2011 [Neuroscience, Clinical Evidence, and the Future of Psychiatric Classification in DSM-5] and abandon the grand plan.
But in that piece, they said…
"In A Research Agenda for DSM-V, we anticipated that these emerging diagnostic and treatment advances would impact the diagnosis and classification of mental disorders faster than what has actually occurred."
…implying that it was still true, but that they hadn’t quite gotten there
yet. And later that year, Dr. Jeffrey Lieberman said in a
Medscape video:
DSM-V promises to be the state of the art in terms of psychiatric diagnoses, and when it was initiated we anticipated that this iteration of the DSM would incorporate biological markers and laboratory-based test results to augment the historical and phenomenologic criteria that traditionally are used to establish psychiatric diagnoses. Sadly, this has proved to be beyond the reach of the current level of evidence for incorporating into this version of the DSM, and it appears that psychiatric diagnoses, which may be rearranged, consolidated, and modified in some ways, will still be based predominantly on symptomatic and historical criteria. However, I am here to tell you that the time is not far off in the future when psychiatric diagnoses of mental disorders and behavioral disturbances will be aided by laboratory-based tests, and this will mark a milestone in the evolution of psychiatric medicine and will occasion an enormous transformation in the accuracy and the reliability of psychiatric diagnoses…
Finally, genetic testing will also come into play. As you probably know, commercial companies already are marketing DNA testing. They provide a "readout" of your genotypes for all of the known coded human genes along with associations with specific diseases in the different organ systems that these correspond to, to the best level of evidence that currently exists. These are not accepted as medically validated and are not used routinely in clinical practice but there is no reason psychiatry cannot begin to use these as other fields of medicine have done. Because all mental disorders will almost certainly prove to be polygenic or multigenic, we will need a gene profile to utilize in term of diagnostic information….
… another set of
not-quite-yet just-around-the-corner comments. And that kind of talk still echos in much of the psychiatric rhetoric. Then on the eve of its release, Dr. Insel bailed out of the DSM-5 in favor of the not yet instantiated RDoC, moving the same kind of thinking next door, but on the same block of the same street [see
Transforming Diagnosis].
I’m not competent to vet Joseph Jay’s science. He seems to be aiming towards the conclusion that there is no genetic loading in Mental Illness which feels to me like an overstated polemic, though I’m not knowledgeable enough to judge with conviction. But I have absolutely no trouble at all thinking that Dr. Lieberman’s alternative, "all mental disorders will almost certainly prove to be polygenic or multigenic" is currently indefensible at face value – particularly after the decade long attempt that followed A Research Agenda for DSM-V. I don’t know of any findings since that book was released that justifies the not-quite-yet just-around-the-corner attitudes still prevalent in the ranks of the NIMH and much of academic psychiatry. They talk as if these now aging hypotheses from that book are still elusive truths in need of confirmation, rather than wishful thinking that has become increasingly unlikely with each passing study. The certainty implied by Dr. Lieberman here [there and everywhere], or Dr. Insel’s certainty with his RDoC or his sponsorship of the Clinical Neuroscience meme [and curriculum project – see the talk that matters…] has outlived credibility.
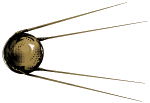 I was excited by the Genome Project too. I think I was as naive as the next guy, thinking it would be a cornucopia of answers. It has turned out to be a first step in a new ballgame we don’t even know how to play – more Sputnik than Star Trek. But it is what it is. It’s a long way around this corner, and we have no clue what we’ll find there. If I had some sermons to preach about all of this, one would be that it’s high time to get all of this out of the guild wars. We’re spending a lot of money and brain power fighting the battle of the guilds, psychology vs psychiatry vs other, and that’s not going to lead us anywhere worth going. This isn’t about guild legitimacy, it’s about Mental Health. But my loudest sermon would be about research funding, particularly by the NIMH. This whole business of special areas that get high priority in funding hasn’t panned out. "Translational Science" didn’t work for mental health as evidenced by how little there has been to "translate." Tom Insel and his staff have no special knowing about what directions we need to move in, and that’s been proven to everyone’s satisfaction. We need to open up the NIMH to our investigators and see what they can come up with on their own, rather than asking them to follow directions set from above.
I was excited by the Genome Project too. I think I was as naive as the next guy, thinking it would be a cornucopia of answers. It has turned out to be a first step in a new ballgame we don’t even know how to play – more Sputnik than Star Trek. But it is what it is. It’s a long way around this corner, and we have no clue what we’ll find there. If I had some sermons to preach about all of this, one would be that it’s high time to get all of this out of the guild wars. We’re spending a lot of money and brain power fighting the battle of the guilds, psychology vs psychiatry vs other, and that’s not going to lead us anywhere worth going. This isn’t about guild legitimacy, it’s about Mental Health. But my loudest sermon would be about research funding, particularly by the NIMH. This whole business of special areas that get high priority in funding hasn’t panned out. "Translational Science" didn’t work for mental health as evidenced by how little there has been to "translate." Tom Insel and his staff have no special knowing about what directions we need to move in, and that’s been proven to everyone’s satisfaction. We need to open up the NIMH to our investigators and see what they can come up with on their own, rather than asking them to follow directions set from above.
I’m still looking for the same thing as this time last year – a broad balance from the NIMH. And I don’t think we’ll ever have it so long as Dr. Insel’s at the helm. He’s in a rut…


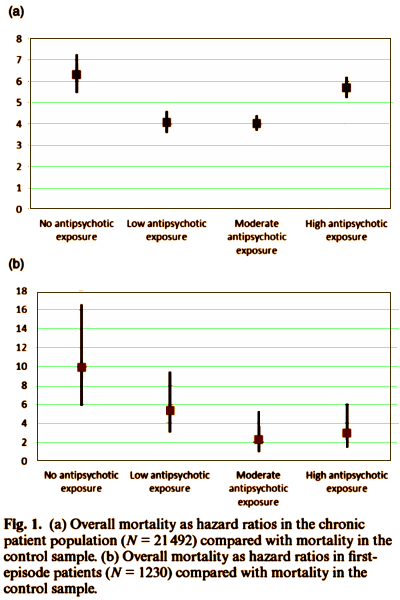
 If you didn’t grow up in the South in the 1940s and 1950s, you couldn’t possible imagine how big this is. That license plate on the right was on the front of many cars [in some places "most cars"] and "The South shall rise again!" was a stock phrase. Even those of us who were dedicated to racial equality and were marching in the streets saw that flag and those slogans, as having some misty-eyed meaning. That was wrong. And many of us figured that out along the way. But for the Legislature of the State of South Carolina to actually do something about it is truly a something I thought I’d never see. There will come a time when this something will be the history that South Carolinians are proud of…
If you didn’t grow up in the South in the 1940s and 1950s, you couldn’t possible imagine how big this is. That license plate on the right was on the front of many cars [in some places "most cars"] and "The South shall rise again!" was a stock phrase. Even those of us who were dedicated to racial equality and were marching in the streets saw that flag and those slogans, as having some misty-eyed meaning. That was wrong. And many of us figured that out along the way. But for the Legislature of the State of South Carolina to actually do something about it is truly a something I thought I’d never see. There will come a time when this something will be the history that South Carolinians are proud of… 
 Some time back, I began to hear a new term – "New Vigil." I thought it was a street drug – slang – because the people where I live were traveling some 50 miles away during the recession to work in the numerous chicken processing plants there [shift work]. So I thought "New Vigil" was a nickname for some new version pep pill they were taking to stay up all night plucking chickens.
Some time back, I began to hear a new term – "New Vigil." I thought it was a street drug – slang – because the people where I live were traveling some 50 miles away during the recession to work in the numerous chicken processing plants there [shift work]. So I thought "New Vigil" was a nickname for some new version pep pill they were taking to stay up all night plucking chickens.


![Gregor Mendel [1822-1884] Gregor Mendel [1822-1884]](http://1boringoldman.com/images/mendel-1.gif)
 Gregor Mendel was an Augustinian Monk in Moravia whose studies of plant inheritance brought such things into the realm of science. My sister’s blue eyes meant that both of our brown-eyed parents carried recessive blue-eye genes. The postman was out of the picture. Mendel’s schemes of inheritance were like that – very precise statistical predictions based on dominant genes. Things stayed pretty clean through Watson and Crick’s images of the double helix DNA structure, even Nirenberg’s nucleotide code sequences. But then things got out of hand – the Genome…
Gregor Mendel was an Augustinian Monk in Moravia whose studies of plant inheritance brought such things into the realm of science. My sister’s blue eyes meant that both of our brown-eyed parents carried recessive blue-eye genes. The postman was out of the picture. Mendel’s schemes of inheritance were like that – very precise statistical predictions based on dominant genes. Things stayed pretty clean through Watson and Crick’s images of the double helix DNA structure, even Nirenberg’s nucleotide code sequences. But then things got out of hand – the Genome…
 I was excited by the Genome Project too. I think I was as naive as the next guy, thinking it would be a cornucopia of answers. It has turned out to be a first step in a new ballgame we don’t even know how to play – more Sputnik than Star Trek. But it is what it is. It’s a long way around this corner, and we have no clue what we’ll find there. If I had some sermons to preach about all of this, one would be that it’s high time to get all of this out of the guild wars. We’re spending a lot of money and brain power fighting the battle of the guilds, psychology vs psychiatry vs other, and that’s not going to lead us anywhere worth going. This isn’t about guild legitimacy, it’s about Mental Health. But my loudest sermon would be about research funding, particularly by the NIMH. This whole business of special areas that get high priority in funding hasn’t panned out. "Translational Science" didn’t work for mental health as evidenced by how little there has been to "translate." Tom Insel and his staff have no special knowing about what directions we need to move in, and that’s been proven to everyone’s satisfaction. We need to open up the NIMH to our investigators and see what they can come up with on their own, rather than asking them to follow directions set from above.
I was excited by the Genome Project too. I think I was as naive as the next guy, thinking it would be a cornucopia of answers. It has turned out to be a first step in a new ballgame we don’t even know how to play – more Sputnik than Star Trek. But it is what it is. It’s a long way around this corner, and we have no clue what we’ll find there. If I had some sermons to preach about all of this, one would be that it’s high time to get all of this out of the guild wars. We’re spending a lot of money and brain power fighting the battle of the guilds, psychology vs psychiatry vs other, and that’s not going to lead us anywhere worth going. This isn’t about guild legitimacy, it’s about Mental Health. But my loudest sermon would be about research funding, particularly by the NIMH. This whole business of special areas that get high priority in funding hasn’t panned out. "Translational Science" didn’t work for mental health as evidenced by how little there has been to "translate." Tom Insel and his staff have no special knowing about what directions we need to move in, and that’s been proven to everyone’s satisfaction. We need to open up the NIMH to our investigators and see what they can come up with on their own, rather than asking them to follow directions set from above.