The campaign against the Black Box Warning has been relentless since it was added to the labeling of antidepressants in 2004. Here are a dozen major articles along the way opposing the Warning:
-
Gibbons RD, Hur K, Bhaumik DK, Mann JJ.
Arch Gen Psychiatry. 2005 Feb;62(2):165-72.
-
Gibbons RD, Hur K, Bhaumik DK, Mann JJ.
Am J Psychiatry. 2006 Nov;163(11):1898-904.
-
Charles B. Nemeroff, Amir Kalali, Martin B. Keller, Dennis S. Charney, Susan E. Lenderts, Elisa F. Cascade, Hugo Stephenson, and Alan F. Schatzberg
Arch Gen Psychiatry. 2007 Apr;64(4):466-472.
-
Nakagawa A, Grunebaum MF, Ellis SP, Oquendo MA, Kashima H, Gibbons RD, Mann JJ.
J Clin Psychiatry. 2007 Jun;68(6):908-16.
-
Gibbons RD, Brown CH, Hur K, Marcus SM, Bhaumik DK, Mann JJ.
Am J Psychiatry. 2007 Jul;164(7):1044-9.
-
Gibbons RD, Brown CH, Hur K, Marcus SM, Bhaumik DK, Erkens JA, Herings RM, Mann JJ.
Am J Psychiatry. 2007 Sep;164(9):1356-63.
-
Brown CH, Wyman PA, Brinales JM, Gibbons RD.
Int Rev Psychiatry. 2007 Dec;19(6):617-31.
-
Gibbons RD, Segawa E, Karabatsos G, Amatya AK, Bhaumik DK, Brown CH, Kapur K, Marcus SM, Hur K, Mann JJ.
Stat Med. 2008 May 20;27(11):1814-33.
-
Barry CL and Busch SH.
Pediatrics. 2010 125[1]:88-95.
-
Gibbons RD, Mann JJ.
Drug Saf. 2011 May 1;34(5):375-95.
-
Robert D. Gibbons, Hendricks Brown, Kwan Hur, John M. Davis, and J. John Mann
Arch Gen Psychiatry. 2012 Jun;69(6):580-587.
-
Christine Y Lu, Fang Zhang , Matthew D Lakoma analyst, Jeanne M Madden, Donna Rusinak, Robert B Penfold, Gregory Simon, Brian K Ahmedani, Gregory Clarke, Enid M Hunkeler, Beth Waitzfelder, Ashli Owen-Smith, Marsha A Raebel, Rebecca Rossom, Karen J Coleman, Laurel A Copeland, Stephen B Soumerai
BMJ. 2014 348:g3596.
Now we have the first of an advertised two-part series on the topic in the PSYCHIATRICNEWS. I was not aware that the American Psychiatric Association [APA] had been so active in its opposition to the Warning in 2004 and going forward:
PSYCHIATRICNEWS
by Mark Moran
12/30/2014
Abstract: This is the first of a two-part series on the 2004 FDA hearings on antidepressants and suicidality in adolescents. Part two will focus on the effect of the antidepressant label warning on prescribing and adolescent health.
From the beginning, and in the decade since, APA has protested the warning, predicting that it would prevent parents from seeking care for their children—and clinicians from prescribing antidepressants—and insisting that by far the greater danger was untreated depression.
‘We are concerned that the publicity surrounding this issue may frighten some parents and discourage them from seeking help for their children,’ child psychiatrist David Fassler, M.D., told the 2004 panel. ‘The most important point that I can make is that the biggest risk for a child with depression is to be left untreated…’
Darrel Regier, M.D., M.P.H., who is the former director of the APA Division of Research and testified at the hearings against the warning, noted that there were in fact no actual suicides among the thousands of teens treated in clinical trials reviewed by the FDA panel. Moreover, the FDA data on suicidal ideation associated with SSRI use was based on spontaneous reports, not on studies prospectively designed to systematically define and identify the incidence of suicidal thoughts or behaviors in teenagers taking the medications.
Regier believes the extraordinary media attention the hearings attracted is a case study in how data pertinent to public health can be obscured or distorted by overinterpretation of spontaneous reporting. “I think it calls into question the FDA’s entire system of spontaneous reporting,” he said. Regier said a promising step is the agency’s adoption, for use in future phase 3 clinical trials, of the Columbia Suicide Severity Rating Scale for any drug that is thought to have some potential for suicidal ideation [including dermatologic drugs such as Accutane].
‘I think the solution is more systematic reporting, which the FDA will start to move toward as the agency begins to have access to electronic health records used by large health systems and HMOs,’ Regier said. ‘This will give the FDA access to phase 4–type data on the long-term effects of medication side effects and associations with suicidal attempts and mortality from all causes.’
It’s unlikely that anyone reading this is neutral on the topic – one that has pervaded our literature almost from the time of the introduction of the SSRIs a quarter of a century ago. Those who support the Black Box Warning rely on individual instances – their own experience as patients and clinicians or the case reports of others. People who oppose the Warning point to studies in populations that show no effect either way on suicide, but confirm that the prescription rate of SSRIs either fell or stopped rising in response to the Warning. It’s hard to find a discussion of this issue that doesn’t also make ad hominem accusations of bias towards the other camp. With that as an introduction, I’m sure not going to revive my own usual arguments, refutations, or accusations. I’d rather talk about an article I read yesterday [sent by literature maven, James O’neill] that seems to fit my take on all of this:
by Fava GA, Guidi J, Rafanelli C, and Sonino N
Psychotherapy and Psychosomatics. 2015 84:1-3.
It’s a short two paged article best read as a narrative rather than viewed in a couple of sound bytes, so I’ll skip any attempt at summary. It’s an article that talks about clinical judgement taking precedence over the tenets of evidence-based medicine. It’s a hard argument to make as it can be instantly met with "How do you know that?" or "But that’s just what you think." And I’ve actually never heard any two people have this argument with even slightly changed minds as a result.
My own opinions are embedded in my narrative. I came into the world equipped for science – aptitude, interest, a father who taught chemistry and geometry. After a degree in mathematics, I headed to medical school intending a medical research career and, in fact, wound up my training in a lab bench based NIH fellowship. I lived and breathed evidence-based medicine [I would like to think I still do]. My first post-training posting was as an general internist in an Air Force hospital overseas [by special invitation from the US government – AKA drafted]. I had been well trained as a clinician, but what I quickly realized was that in training, I had always been located somewhere in the hierarchy of a team of some sort, and when it was just me and a patient, medicine was a different game altogether. What Giovanni Fava is calling clinical judgement is as good a way as any to describe the difference.
I think of that three years as the most important and intense period of my own medical training. I became so interested in the individual illnesses and unique experience of my patients that I returned to a psychiatry residency and added a psychoanalytic training program that ran in parallel. My post academic career was as an individual psychotherapist in a world where clinical judgement was on the front burner of every day.
There was another important and intense period of my own medical training after my psychiatry residency. I directed a psychiatry training program for a decade before leaving for practice during the storm in psychiatry that followed the coming of the DSM-III. And I noticed something in my dealing with my residents during those days. I often found myself preaching the gospel of evidence-based medicine – quoting studies, handing out articles, sending a resident to the library. But at other times, my sermon was equally passionate, but was much more in the range of Fava’s clinical judgement. When I noticed that contrast, I quickly realized that the theme of my message depended on which resident I was talking to, and what I thought that particular resident needed to learn. The point being that I don’t slightly see evidence-based medicine and clinical judgement as a dichotomy – in opposition. Medicine as I know it is practiced at the interface of group data and unique human experience [including my own].
So how do I relate this little discourse to the issue of prescribing SSRIs to adolescents? First, I object to the "untreated depression" argument out of hand. The "depression" on the table here is, in my view, a symptom – not a disease. And there is no formula I know that says that the "treatment" of "adolescent depression" is "SSRIs" or, for that matter, medication. I see the drugs as symptomatic medications that may, at times, be helpful in the "treatment of a particular depressed adolescent." Second, I have no question that the SSRIs can occasionally cause a unique syndrome [called Akathisia by David Healy] characterized by agitation, aggression, disinhibition, and intensely lethal thought. I know that’s true because I did it to someone:
I saw a seventeen year old who was very depressed with good reason. His mother had remarried a man who was a retired Drill Sergeant [really] and this kid and his step-father were oil and water – at war. The boy had an impending interview for a training program that would’ve paid his way to move 200 miles away and study something he had dreamed of all his life. His mother and I were both worried that he was so depressed that he wouldn’t "pass" the interview. So I prescribed an SSRI. In a few days, he developed the full Akathisia syndrome in spades, including being intensely suicidal [for the first time]. His mother stopped the medication, didn’t leave his side, and it cleared within 48 hours.
I’ve since seen other cases of Akathisia and know of two completed suicides from SSRIs, fortunately none cases of my own. But that first case was from around the time of the Black Box Warning and I hadn’t been warned. Worse, I hadn’t warned them. So for me, this issue started in the realm of evidence-based medicine, something I saw in person, and became part of my subsequent clinical judgement. Would I prescribe an SSRI to an adolescent now? I would and have done on some occasions, but with more than plenty of warnings, and only to those I was absolutely sure were going to be closely watched by someone I had talked to myself.
When I look at that dozen articles I listed above, or read what Dr. Regier has to say, I’m aware that I’m a total snob. There’s not anyone that I would consider to be a clinician on the bylines of the articles on the list, or speaking in the PSYCHIATRICNEWS article. They’re Statisticians, Academicians, Researchers, or Administrative types. Even putting aside all the Conflict of Interest and Methodological issues in those papers [which are prodigious], I’m sure that I still wouldn’t change my mind about this no matter how long that list of population studies gets. And, by the way, I sense that the PSYCHIATRICNEWS article is a harbinger of yet another assault on the Black Box Warning [as if what it says on a package insert defines the truth].
I personally take this issue as a microcosm of a much more general point. I most respect the opinions of people who are deeply steeped in the findings of evidence-based medicine, but who remain immersed in clinical medicine throughout their careers [and show the tell-tale signs of having developed clinical judgement]. I don’t think that’s a tall order – more a baseline requirement. Back in 1980, the people who brought the DSM-III revolution in psychiatry to my own neck of the woods saw themselves as the prophets of evidence-based medicine. I sure didn’t object to that, but they were specifically disdainful of anything that slightly smacked of clinical judgement [as in "How do you know that?" or "But that’s just what you think" ad nauseum]. They were followed by a second wave who were even worse, in the range of contemptuous – the generation that became what we now call the KOLs [some of whom inhabit those author bylines above in the flesh]. They came at a time when psychiatry may have needed an encounter over its reliance on matters subjective, but in the process they turned clinical judgement into an object of ridicule rather than one for examination and inquiry.
There’s a strong pressure pushing this same trend in medicine in general – simplifying medical practice into the rote application of large group findings to the care of individual patients based on averages and statistics. That pressure comes from third party carriers, pharmaceutical companies, hospital conglomerates, governments, even professional organizations, etc. – anyone who tries to quantitatively deal with the healthcare of populations. So the amount of allowed contact, the access to diagnostic tests, the available treatments, just about everything is regulated based on algorithms and guidelines derived from large group studies. Meanwhile, patients increasingly clamor for individual attention, and are driven to distraction along with their doctors by the hoops they have to jump through when they get sick. Dr. Regier’s comment is typical of this particular mindset, "I think the solution is more systematic reporting, which the FDA will start to move toward as the agency begins to have access to electronic health records used by large health systems and HMOs."
So while Dr. Fava’s comments appear in a mental health journal, I think they can be generalized to all of healthcare:
The conceptual model that has generated EBM and guidelines clashes with clinical reality and fosters a dichotomy between medical science and clinical judgment. EBM has certainly made an important contribution to questioning unsubstantiated therapeutic claims. The time has come, however, to become more aware of its considerable limitations, including overall reductionism, disregard of patient-physician relationships and patient preferences, and insufficient consideration of problems related to financial conflicts of interest. As an increasing body of literature indicates, EBM offers only a restrictive interpretation of the scientific approach to clinical practice.


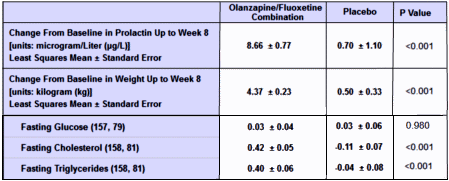
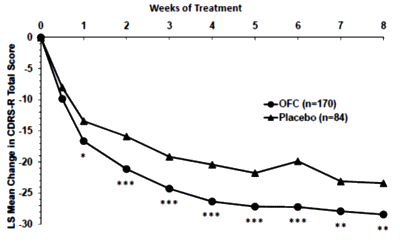
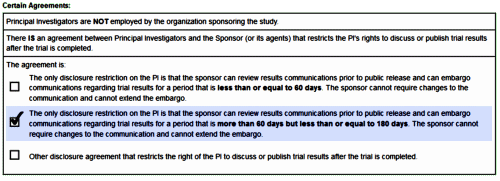
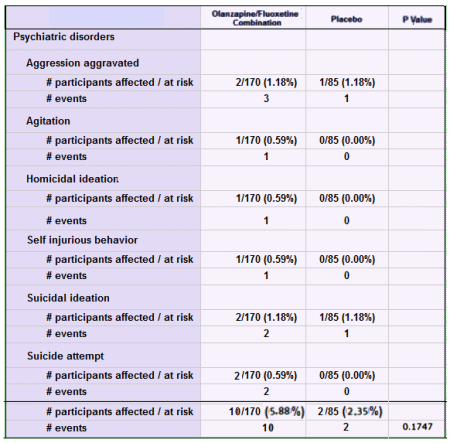
 Black Box Warning. Except for Prozac®, they’re advocating off-label prescribing for marginal efficacy at best. There’s unlikely much push from PHARMA now that the drugs are off-patent. In spite of the limitations, they’re still prescribing at a moderate pace. Seems to me they’d let this sleeping dog lie…
Black Box Warning. Except for Prozac®, they’re advocating off-label prescribing for marginal efficacy at best. There’s unlikely much push from PHARMA now that the drugs are off-patent. In spite of the limitations, they’re still prescribing at a moderate pace. Seems to me they’d let this sleeping dog lie…


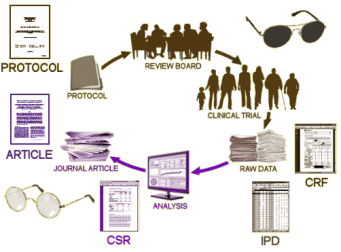
 I’ve never prescribed Lyrica®, and only refilled Neurontin®. The thing I’ve noticed is that patients are reticent to stop Neurontin®, and I suspect it is because it has a mild Benzo-like antianxiety effect. The problem with this system is contained in this letter. "This runs contrary to the government’s established policy of rewarding additional research by granting a second medical use patent". That’s built on the notion that pharmaceutical companies should be rewarded in return for developing new drugs for the betterment of our patient’s lives. They’ve turned that into a mechanism to make gajillions off of medications that have a minimal effect [if that] and then sell that effect to the public that clamors for the drug so they can be like the pretty lady on the television dancing her way through an exotic vacation.
I’ve never prescribed Lyrica®, and only refilled Neurontin®. The thing I’ve noticed is that patients are reticent to stop Neurontin®, and I suspect it is because it has a mild Benzo-like antianxiety effect. The problem with this system is contained in this letter. "This runs contrary to the government’s established policy of rewarding additional research by granting a second medical use patent". That’s built on the notion that pharmaceutical companies should be rewarded in return for developing new drugs for the betterment of our patient’s lives. They’ve turned that into a mechanism to make gajillions off of medications that have a minimal effect [if that] and then sell that effect to the public that clamors for the drug so they can be like the pretty lady on the television dancing her way through an exotic vacation.