Posted on Sunday 24 April 2016
 Gaining perspective on a history you lived through might be the hardest thing of all. How you saw it then and how it looks to you and to others now create a ciphered dreamscape not so easily parsed. Was Janis Joplin a symbol of freedom and liberation or an early slave to a coming opioid epidemic? When you’re passed on the highway by a roaring motorcycle cavalcade, is it the marauding Hell’s Angels or a bunch of Orthopedic Surgeons out for a weekend spin on their gajillion dollar Harleys? It’s the stuff in between, the story of the transition, that often holds the key to understanding. That’s certainly true in a psychotherapy that aims to find how some childhood situation morphs into a rigid maladaptive character trait that wreaks havoc with an adult life. There was such a transition in the world of psychopharmacology that I basically slept through [mid 1980s through the mid 2000s] and I’m still at trying to fill it in – it’s slow going.
Gaining perspective on a history you lived through might be the hardest thing of all. How you saw it then and how it looks to you and to others now create a ciphered dreamscape not so easily parsed. Was Janis Joplin a symbol of freedom and liberation or an early slave to a coming opioid epidemic? When you’re passed on the highway by a roaring motorcycle cavalcade, is it the marauding Hell’s Angels or a bunch of Orthopedic Surgeons out for a weekend spin on their gajillion dollar Harleys? It’s the stuff in between, the story of the transition, that often holds the key to understanding. That’s certainly true in a psychotherapy that aims to find how some childhood situation morphs into a rigid maladaptive character trait that wreaks havoc with an adult life. There was such a transition in the world of psychopharmacology that I basically slept through [mid 1980s through the mid 2000s] and I’m still at trying to fill it in – it’s slow going.
Within a year of the introduction of chlorpromazine, a National Academy of Sciences grant led to a conference, chaired by Ralph Gerard and Jonathan Cole, aimed at establishing the appropriate evaluative methods for the new pharmacotherapies… There was a recognition that rating scales and randomized controlled trials were needed…[see Recommendations for reporting studies of psychiatric drugs]… further funds were forthcoming from Congress — more than could be utilized by the center. In addition, following the success of chlorpromazine, pharmaceutical companies flooded the market with copycat drugs and approached investigators to test them. These events led Cole to set up in 1959 a clinical committee chaired by Henry Brill to assemble interested clinicians in order to standardize evaluative methods in clinical studies and to avoid duplication of research efforts." Brill recruited … to serve on a steering group for what became The Early Clinical Drug Evaluation Unit [ECDEU]. The unit’s task was to study the safety of new drugs, to find their appropriate dose ranges, and to look for appropriate clinical niches. It was hoped that federal funding would confer independence on the investigators.
One of the major undertakings of the ECDEU group was the development of standardized clinical trial protocols, agreed methods of coding information, and rating scales such as Hollister and John Overall’s Brief Psychiatric Raring Scale [BPRS]. The global assessment scales, which have been a mainstay of clinical trials ever since, were developed by ECDEU. In collaboration with the NIMH, a centralized computerized system [BLIPS] was set up to collate information. These efforts were aimed in part at controlling the potential excesses of the pharmaceutical industry. But there was also a hope that clinical trials could be channeled along development routes that would yield objective and reliable data that could benefit both clinicians and pharmaceutical companies.
In collaboration with The George Washington University Biometric Laboratory, the ECDEU Standard Reporting System has been made available to any investigator interested in conducting clinical trials, whether federally grant supported or not. To utilize these services, the investigator is requested to:
The operational criteria embodied in DSM-III were born into a world different from the one in which they were conceived. Richard Nixon’s election in 1968 might have led to a demise of disinterested research in any event, but the administration also faced a health budget that was burgeoning alarmingly. The Vietnam War had led to an economic crisis in 1968, aggravated several years later by the oil crisis. The government began to cut back on funding for the National institutes of Health. Sensing the change, Jonathan Cole left the Psychopharmacology Research Center [PRC] and returned to clinical practice. The NIMH research budget declined by $5 million from 1969 to 1976. Grants from ECDEU came to an end in 1975. By 1980 state funds for research had dried up. Independent clinical research was over, although since "science" at the NIMH was untouched few realized it…In 1977, Jimmy Carter established a presidential commission on mental health. This recommended greater attention to the psychiatric needs of children and minorities, support for research and in particular for epidemiology, the development of a specific plan for treating the chronically mentally ill, and the establishment of methods for monitoring the performance of the mental health services. These initiatives were embodied in the Mental Health Systems Act, which was signed into law shortly before Carter left office. This Canute-like bill faced a strong adverse tide. Two months later, the new Reagan government recommended an interruption of all mental health grant programs in research and training. This Republican administration had come into office committed to lowering taxes, deregulation, decreasing federal control, and increasing the states’ authority. The new act was dismantled. Federal care and social security support for the chronically mentally ill went into a sharp decline, and the continuing increase in health care budgets provided the matrix for the birth of managed care…
By the mid-1970s the ECDEU program was failing. Funds had dried up. Out of the ashes of ECDEU arose a superficially similar body, the New Clinical Drug Evaluation Unit [NECDEU], but one with a very different character. This was a marketplace where companies hired clinical investigators. Previously researchers had told industry what needed to be done, but now companies did not have to approach investigators to design their trials for them, compile the statistics, or write the papers. The formulas for clinical trials that the ECDEU investigators had put together to contain the pharmaceutical industry became a petard on which psychiatry was hoist. Armed with off-the-shelf protocols, companies sought out those researchers who were prepared to do the work that suited a commercial agenda. A process had begun that led to the analysis of trial results within the company and thereafter to the writing up of the results by company personnel. Senior clinical investigators now might be used as figureheads on papers or for presentations at academic meetings, but the clinical presence was increasingly becoming ornamental rather than substantial. They were merely figureheads for studies conducted by relatively untrained nonmedical personnel and in some cases the patients did not exist…


 Wikipedia
Wikipedia
 Americans, therefore, should hardly be surprised so many Britons resent distant and unaccountable EU governance. On this side of the Atlantic, we readily understand that sovereignty resides constitutionally in “we, the people,” not in the federal government. At a time of dwindling confidence that we have control over our own government, the idea of sharing sovereignty internationally, thereby diluting it even further, is decidedly as unattractive here as in Britain.
Americans, therefore, should hardly be surprised so many Britons resent distant and unaccountable EU governance. On this side of the Atlantic, we readily understand that sovereignty resides constitutionally in “we, the people,” not in the federal government. At a time of dwindling confidence that we have control over our own government, the idea of sharing sovereignty internationally, thereby diluting it even further, is decidedly as unattractive here as in Britain.  What I learned living in the UK for three years is that two things were not to ever be understood by yours truly – British Politics and Cricket.
What I learned living in the UK for three years is that two things were not to ever be understood by yours truly – British Politics and Cricket.  And since I was there, they’ve added the European Union to the mix, so now I double-dog don’t understand. So Obama is headed to London, and he wants the UK to stay in the EU whereas John Bolton, despised by me from his Bush UN Ambassador days as a noted neoconservative opposed to the UN, is fanning Brexit [BRitish EXIT] flames on the side of the Queen. Who can sort this out? Well, the British people will soon have the chance to vote. The "INs" are in the lead, but the polls have gone back and forth.
And since I was there, they’ve added the European Union to the mix, so now I double-dog don’t understand. So Obama is headed to London, and he wants the UK to stay in the EU whereas John Bolton, despised by me from his Bush UN Ambassador days as a noted neoconservative opposed to the UN, is fanning Brexit [BRitish EXIT] flames on the side of the Queen. Who can sort this out? Well, the British people will soon have the chance to vote. The "INs" are in the lead, but the polls have gone back and forth.
 Let me start over. There’s a whole line of thinking that conceptualizes depression as if it’s an epidemic medical disease. The logic begins with some allusion to the prevalence in the population and the notion that it is rapidly increasing, then progresses to some kind of estimate of the cost in lost productivity and things like over-use of medical services etc. The end point of this logic train is that we need early detection, rapid treatment [antidepressants], and better drugs than the ones we currently have.
Let me start over. There’s a whole line of thinking that conceptualizes depression as if it’s an epidemic medical disease. The logic begins with some allusion to the prevalence in the population and the notion that it is rapidly increasing, then progresses to some kind of estimate of the cost in lost productivity and things like over-use of medical services etc. The end point of this logic train is that we need early detection, rapid treatment [antidepressants], and better drugs than the ones we currently have.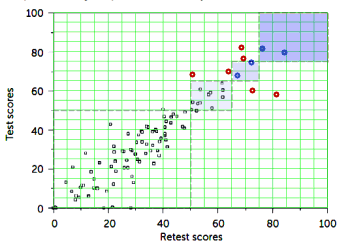
 The ever-sensible British Neuroscientist we know as
The ever-sensible British Neuroscientist we know as